Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation - PubMed (original) (raw)
Review
Cytochrome P450 epoxygenases, soluble epoxide hydrolase, and the regulation of cardiovascular inflammation
Yangmei Deng et al. J Mol Cell Cardiol. 2010 Feb.
Abstract
The cytochrome P450 (CYP) epoxygenase enzymes CYP2J and CYP2C catalyze the epoxidation of arachidonic acid to epoxyeicosatrienoic acids (EETs), which are rapidly hydrolyzed to dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH). It is well-established that CYP epoxygenase-derived EETs possess potent vasodilatory effects; however, the cellular effects of EETs and their regulation of various inflammatory processes have become increasingly appreciated in recent years, suggesting that the role of this pathway in the cardiovascular system extends beyond the maintenance of vascular tone. In particular, CYP epoxygenase-derived EETs inhibit endothelial activation and leukocyte adhesion via attenuation of nuclear factor-kappaB activation, inhibit hemostasis, protect against myocardial ischemia-reperfusion injury, and promote endothelial cell survival via modulation of multiple cell signaling pathways. Thus, the CYP epoxygenase pathway is an emerging target for pharmacological manipulation to enhance the cardiovascular protective effects of EETs. This review will focus on the role of the CYP epoxygenase pathway in the regulation of cardiovascular inflammation and (1) describe the functional impact of CYP epoxygenase-derived EET biosynthesis and sEH-mediated EET hydrolysis on key inflammatory process in the cardiovascular system, (2) discuss the potential relevance of this pathway to pathogenesis and treatment of cardiovascular disease, and (3) identify areas for future research.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
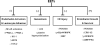
Figure 1. Overview of CYP Epoxygenase Pathway Metabolism
Upon cPLA2 activation, arachidonic acid is released and oxidatively metabolized by cyclooxygenases (COX), lipoxygenases (LOX) and cytochromes P450 (CYP) into biologically active eicosanoids. This review focuses on the CYP epoxygenase pathway. Isoforms from the CYP2J and CYP2C subfamilies synthesize four epoxyeicosatrienoic acid (EET) regioisomers (11,12-EET is shown), which are subsequently incorporated into membrane phospholipids or rapidly hydrolyzed by soluble epoxide hydrolase (sEH) into their corresponding diol dihydroxyeicosatrienoic acid (DHET) metabolites (11,12-DHET is shown). The DHETs generally possess less potent biological activity than EETs.
Figure 2. Overview of the Cardiovascular Protective Effects of CYP Epoxygenase-Derived EETs
A series of recent studies have demonstrated that CYP epoxygenase-derived EETs possess potent cardiovascular protective effects including inhibition of endothelial activation and leukocyte adhesion, inhibition of hemostasis, attenuation of myocardial and endothelial ischemia-reperfusion (I/R) injury, and promotion of endothelial cell survival.
Similar articles
- Endothelial CYP epoxygenase overexpression and soluble epoxide hydrolase disruption attenuate acute vascular inflammatory responses in mice.
Deng Y, Edin ML, Theken KN, Schuck RN, Flake GP, Kannon MA, DeGraff LM, Lih FB, Foley J, Bradbury JA, Graves JP, Tomer KB, Falck JR, Zeldin DC, Lee CR. Deng Y, et al. FASEB J. 2011 Feb;25(2):703-13. doi: 10.1096/fj.10-171488. Epub 2010 Nov 8. FASEB J. 2011. PMID: 21059750 Free PMC article. - Influence of inflammation on cardiovascular protective effects of cytochrome P450 epoxygenase-derived epoxyeicosatrienoic acids.
Shahabi P, Siest G, Visvikis-siest S. Shahabi P, et al. Drug Metab Rev. 2014 Feb;46(1):33-56. doi: 10.3109/03602532.2013.837916. Epub 2013 Sep 16. Drug Metab Rev. 2014. PMID: 24040964 Review. - Role of cytochrome P450-epoxygenase and soluble epoxide hydrolase in the regulation of vascular response.
Nayeem MA, Geldenhuys WJ, Hanif A. Nayeem MA, et al. Adv Pharmacol. 2023;97:37-131. doi: 10.1016/bs.apha.2022.12.003. Epub 2023 Jan 2. Adv Pharmacol. 2023. PMID: 37236764 - The cytochrome P450 epoxygenase pathway regulates the hepatic inflammatory response in fatty liver disease.
Schuck RN, Zha W, Edin ML, Gruzdev A, Vendrov KC, Miller TM, Xu Z, Lih FB, DeGraff LM, Tomer KB, Jones HM, Makowski L, Huang L, Poloyac SM, Zeldin DC, Lee CR. Schuck RN, et al. PLoS One. 2014 Oct 13;9(10):e110162. doi: 10.1371/journal.pone.0110162. eCollection 2014. PLoS One. 2014. PMID: 25310404 Free PMC article. - The roles of CYP450 epoxygenases and metabolites, epoxyeicosatrienoic acids, in cardiovascular and malignant diseases.
Xu X, Zhang XA, Wang DW. Xu X, et al. Adv Drug Deliv Rev. 2011 Jul 18;63(8):597-609. doi: 10.1016/j.addr.2011.03.006. Epub 2011 Apr 6. Adv Drug Deliv Rev. 2011. PMID: 21477627 Review.
Cited by
- Cell biology of ischemia/reperfusion injury.
Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Kalogeris T, et al. Int Rev Cell Mol Biol. 2012;298:229-317. doi: 10.1016/B978-0-12-394309-5.00006-7. Int Rev Cell Mol Biol. 2012. PMID: 22878108 Free PMC article. Review. - Deficiency of Soluble Epoxide Hydrolase Protects Cardiac Function Impaired by LPS-Induced Acute Inflammation.
Samokhvalov V, Jamieson KL, Darwesh AM, Keshavarz-Bahaghighat H, Lee TYT, Edin M, Lih F, Zeldin DC, Seubert JM. Samokhvalov V, et al. Front Pharmacol. 2019 Jan 14;9:1572. doi: 10.3389/fphar.2018.01572. eCollection 2018. Front Pharmacol. 2019. PMID: 30692927 Free PMC article. - Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma.
Ono E, Dutile S, Kazani S, Wechsler ME, Yang J, Hammock BD, Douda DN, Tabet Y, Khaddaj-Mallat R, Sirois M, Sirois C, Rizcallah E, Rousseau E, Martin R, Sutherland ER, Castro M, Jarjour NN, Israel E, Levy BD; National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. Ono E, et al. Am J Respir Crit Care Med. 2014 Oct 15;190(8):886-97. doi: 10.1164/rccm.201403-0544OC. Am J Respir Crit Care Med. 2014. PMID: 25162465 Free PMC article. - Pro-atherogenic role of smooth muscle Nox4-based NADPH oxidase.
Tong X, Khandelwal AR, Wu X, Xu Z, Yu W, Chen C, Zhao W, Yang J, Qin Z, Weisbrod RM, Seta F, Ago T, Lee KS, Hammock BD, Sadoshima J, Cohen RA, Zeng C. Tong X, et al. J Mol Cell Cardiol. 2016 Mar;92:30-40. doi: 10.1016/j.yjmcc.2016.01.020. Epub 2016 Jan 23. J Mol Cell Cardiol. 2016. PMID: 26812119 Free PMC article. - Genetic variants in epoxyeicosatrienoic acid processing and degradation pathways are associated with gestational diabetes mellitus.
Lai S, Yan D, Xu J, Yu X, Guo J, Fang X, Tang M, Zhang R, Zhang H, Jia W, Luo M, Hu C. Lai S, et al. Nutr J. 2023 Jun 28;22(1):31. doi: 10.1186/s12937-023-00862-9. Nutr J. 2023. PMID: 37370090 Free PMC article.
References
- Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679–82. - PubMed
- Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–60. - PubMed
- Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–85. - PubMed
- Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–62. - PubMed
- Rendic S, Di Carlo FJ. Human cytochrome P450 enzymes: a status report summarizing their reactions, substrates, inducers, and inhibitors. Drug Metab Rev. 1997;29:413–580. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources