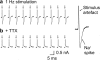Examining size-strength relationships at hippocampal synapses using an ultrastructural measurement of synaptic release probability - PubMed (original) (raw)
Examining size-strength relationships at hippocampal synapses using an ultrastructural measurement of synaptic release probability
Tiago Branco et al. J Struct Biol. 2010 Nov.
Abstract
Release probability (p(r)) is a fundamental presynaptic parameter which is critical in defining synaptic strength. Knowledge of how synapses set and regulate their p(r) is a fundamental step in understanding synaptic transmission and communication between neurons. Despite its importance, p(r) is difficult to measure directly at single synapses. One important strategy to achieve this has relied on the application of fluorescence-based imaging methods, but this is always limited by the lack of detailed information on the morphological and structural properties of the individual synapses under study, and thus precludes an investigation of the relationship between p(r) and synaptic anatomy. Here we outline a powerful methodology based on using FM-styryl dyes, photoconversion and correlative ultrastructural analysis in dissociated hippocampal cultured neurons, which provides both a direct readout of p(r) as well as nanoscale detail on synaptic organization and structure. We illustrate the value of this approach by investigating, at the level of individual reconstructed terminals, the relationship between release probability and defined vesicle pools. We show that in our population of synapses, p(r) is highly variable, and while it is positively correlated with the number of vesicles docked at the active zone it shows no relationship with the total number of synaptic vesicles. The lack of a direct correlation between total synaptic size and performance in these terminals suggests that factors other than the absolute magnitude of the synapse are the most important determinants of synaptic efficacy.
Copyright © 2009 Elsevier Inc. All rights reserved.
Figures
Supplementary Fig. 1
Reliability of field stimulation. (a) Consecutive traces of a whole-cell recording in voltage-clamp mode during field stimulation (1 s interval between traces). Each trace shows an artefactual component arising from the stimulation followed by a prominent unclamped sodium spike. (b) In the presence of TTX, this spike is blocked, confirming that it arises from the activation of voltage-gated Na+ channels.
Fig. 1
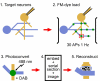
A schematic illustrating the steps in making ultrastructural measurements of pr. Cultured neurons are targeted (and, for example, filled with fluorescent dye to aid correlative EM) (step 1) before being loaded with FM1-43 using field stimulation (30 APs, 1 Hz) (step 2). After fixation, FM-dye is photoconverted to an electron-dense form in the presence of DAB (step 3) before being embedded, serially sectioned and imaged (step 4). Presynaptic terminals can then be fully reconstructed, permitting a count of the total number of recycled vesicles relative to the defined loading stimulus, and giving a measure of release probability (step 5).
Fig. 2
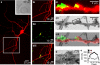
An example of the correlative approach for readout of pr at a specific presynaptic terminal. (a) Target hippocampal neuron filled with red Alexa dye. Inset shows brightfield image of the region of interest. (bi–biii) Detail of white square region in (a) showing red dye-filled processes (bi), presynaptic terminals labelled with FM1-43 (bii) and a composite overlay (biii). Yellow arrow indicates a putative presynaptic terminal contact site with the red neuron. (c) Detail on contact site in (b). (ci) FM1-43 and red Alexa dye composite. (cii) Equivalent region seen in a low magnification electron micrograph. (ciii) Overlay of (ci) and (cii). (d) Target synapse (indicated by box in (cii)) with photoconverted vesicles (dark lumen) and non-photoconverted vesicles (clear lumen). (e) A photoconverted vesicle can be readily discriminated from a non-photoconverted vesicle with a linescan of optical density.
Fig. 3
Three-dimensional reconstruction of synapse in Fig. 2 based on a complete section series. Dark vesicles (18) are photoconverted. Non-photoconverted vesicles appear semi-transparent. Release probability is estimated at 0.6. (Bottom) Vesicles in the five sections on which reconstruction is based.
Fig. 4
A correlative ultrastructural approach offers additional information to that available by fluorescence. (ai) DIC image of target region, (aii) target neuron filled with red-dye. (b) Detail of region in (a) showing: (bi) overlay of putative postsynaptic neuron (red) and presynaptic contact points (green). (bii) Equivalent region seen in a low magnification electron micrograph. (biii) Composite overlay of (bi) and (bii). (c) Detail of three synapses marked with arrows in (b). Ultrastructural investigation reveals the synapses have shared axonal and dendritic processes. (di) A target region with two clearly-defined FM1-43 puncta and processes of a red dye-filled neuron. (dii) Overlay of fluorescence and low magnification electron micrograph. (ei and eii) Synaptic regions marked by boxes in (d). The ultrastructural detail reveals that presynaptic terminals ‘1’ and ‘3’ share the same axon, but have different target dendritic compartments. Note: (di) shows a single punctum on the left (arrow) but the electron micrograph in (ei) confirms the presence of two (‘1’ and ‘2’) terminals in close proximity within this region. (f) Schematic summary of synapse arrangement.
Fig. 5
Ultrastructural measurement of pr and relationship to anatomically defined vesicle populations. (a) Summary pr distribution plot for synapses (n = 20) from three cultures. Values are based on ultrastructural measurements using the approach outlined in this paper. (b) Scatter plot showing pr against docked vesicle pool size. These parameters are significantly correlated (R = 0.54, P = 0.03, Pearson correlation). The average number of docked vesicles was 5.8 ± 0.9, similar to the value reported in a previous ultrastructural study in cultured hippocampal neurons (4.6 ± 3.0, Schikorski and Stevens, 1997). (c) Scatter plot for pr against total vesicle pool size reveals no significant relationship (R = −0.16, P = 0.50, Pearson correlation). (d) Four examples of reconstructed synapses showing total vesicle count and the number of photoconverted vesicles. bottom left, nine consecutive serial sections for reconstructed synapse on top left. (e)-(f) demonstrating the variability in recycling pool fraction of total pool using FM-dye-photoconversion-EM methods. (e) Two examples of fully reconstructed synapses with very different total vesicle pool recycling fractions. (f) Scatter plot of the recycling fraction of n = 13 synapses against their total pool size.
Similar articles
- Ultrastructural readout of functional synaptic vesicle pools in hippocampal slices based on FM dye labeling and photoconversion.
Marra V, Burden JJ, Crawford F, Staras K. Marra V, et al. Nat Protoc. 2014;9(6):1337-47. doi: 10.1038/nprot.2014.088. Epub 2014 May 15. Nat Protoc. 2014. PMID: 24833172 - Constitutive sharing of recycling synaptic vesicles between presynaptic boutons.
Darcy KJ, Staras K, Collinson LM, Goda Y. Darcy KJ, et al. Nat Neurosci. 2006 Mar;9(3):315-21. doi: 10.1038/nn1640. Epub 2006 Feb 5. Nat Neurosci. 2006. PMID: 16462738 - Synapsin selectively controls the mobility of resting pool vesicles at hippocampal terminals.
Orenbuch A, Shalev L, Marra V, Sinai I, Lavy Y, Kahn J, Burden JJ, Staras K, Gitler D. Orenbuch A, et al. J Neurosci. 2012 Mar 21;32(12):3969-80. doi: 10.1523/JNEUROSCI.5058-11.2012. J Neurosci. 2012. PMID: 22442064 Free PMC article. - Mechanisms of synaptic vesicle recycling illuminated by fluorescent dyes.
Cousin MA, Robinson PJ. Cousin MA, et al. J Neurochem. 1999 Dec;73(6):2227-39. doi: 10.1046/j.1471-4159.1999.0732227.x. J Neurochem. 1999. PMID: 10582580 Review. - Nanoscale Organization of Vesicle Release at Central Synapses.
Gramlich MW, Klyachko VA. Gramlich MW, et al. Trends Neurosci. 2019 Jun;42(6):425-437. doi: 10.1016/j.tins.2019.03.001. Trends Neurosci. 2019. PMID: 31176424 Free PMC article. Review.
Cited by
- Synaptic spinules are reliable indicators of excitatory presynaptic bouton size and strength and are ubiquitous components of excitatory synapses in CA1 hippocampus.
Gore A, Yurina A, Yukevich-Mussomeli A, Nahmani M. Gore A, et al. Front Synaptic Neurosci. 2022 Aug 11;14:968404. doi: 10.3389/fnsyn.2022.968404. eCollection 2022. Front Synaptic Neurosci. 2022. PMID: 36032419 Free PMC article. - Common strength and localization of spontaneous and evoked synaptic vesicle release sites.
Loy K, Welzel O, Kornhuber J, Groemer TW. Loy K, et al. Mol Brain. 2014 Apr 2;7:23. doi: 10.1186/1756-6606-7-23. Mol Brain. 2014. PMID: 24694031 Free PMC article. - Synaptic counts approximate synaptic contact area in Drosophila.
Barnes CL, Bonnéry D, Cardona A. Barnes CL, et al. PLoS One. 2022 Apr 4;17(4):e0266064. doi: 10.1371/journal.pone.0266064. eCollection 2022. PLoS One. 2022. PMID: 35377898 Free PMC article. - Release probability of hippocampal glutamatergic terminals scales with the size of the active zone.
Holderith N, Lorincz A, Katona G, Rózsa B, Kulik A, Watanabe M, Nusser Z. Holderith N, et al. Nat Neurosci. 2012 Jun 10;15(7):988-97. doi: 10.1038/nn.3137. Nat Neurosci. 2012. PMID: 22683683 Free PMC article. - Development of Parvalbumin-Expressing Basket Terminals in Layer II of the Rat Medial Entorhinal Cortex.
Berggaard N, Bjerke IE, B Paulsen AE, Hoang L, T Skogaker NE, Witter MP, van der Want JJL. Berggaard N, et al. eNeuro. 2018 Jun 26;5(3):ENEURO.0438-17.2018. doi: 10.1523/ENEURO.0438-17.2018. eCollection 2018 May-Jun. eNeuro. 2018. PMID: 29951577 Free PMC article.
References
- Aravanis A.M., Pyle J.L., Tsien R.W. Single synaptic vesicles fusing transiently and successively without loss of identity. Nature. 2003;423:643–647. - PubMed
- Betz W.J., Bewick G.S. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science. 1992;255:200–203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- BB/F018371/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT 084357MF/WT_/Wellcome Trust/United Kingdom
- BB/F018371/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Research Materials