Hematopoietic cell transplantation for autoimmune disease: updates from Europe and the United States - PubMed (original) (raw)
Review
Hematopoietic cell transplantation for autoimmune disease: updates from Europe and the United States
Keith M Sullivan et al. Biol Blood Marrow Transplant. 2010 Jan.
Abstract
Considerable advances have been made in our understanding of the immunobiology of autoimmune disease and its treatment with hematopoietic cell transplantation (HCT). In autoimmune disorders, the reconstituted immune system following lymphoablation and autologous HCT yields qualitative changes in immune defects and modifications in adaptive immune responses. Seminal experiments in animals demonstrated that allogeneic or autologous HCT could prevent progression or reverse organ damage from inherited (genetic) or acquired (antigen induced) autoimmune diseases. Convincing animal and clinical data now show that after HCT, the immune system is normalized and "reset". Following autologous transplantation, this resetting occurs via repertoire replacement. It is currently being studied whether and to what extent suppression of inflammation after HCT is due to reregulation of function or due to the eradication of disease associated T and/or B cell populations. There are now a number of published clinical reports with sufficient follow-up for determinations of safety and efficacy of HCT for autoimmune diseases. On behalf of colleagues in the European League Against Rheumatism (EULAR) and the European Group for Blood and Marrow Transplantation (EBMT), we review the experience with more than 1000 transplants for autoimmune disease in Europe along with the three major multinational randomized trials in for systemic sclerosis (SSc, the ASTIS study), multiple sclerosis (MS, the ASTIMS study), and Crohn's disease (CD, the ASTIC study). Completed phase II studies in the USA of transplantation for severe SSc, SLE and MS yield promising results. For individuals with SSc, there is dramatic improvement/resolution of dermal fibrosis and stabilization/improvement of pulmonary dysfunction reported up to 8 years after lymphoablative conditioning and autologous HCT. Currently, randomized phase III studies are recruiting subjects in the USA with SSc, MS and CD. In addition, 9 other phase I and II trials in the USA are recruiting patients with autoimmune diseases for nonmyeloablative transplants from allogeneic stem cell donors. Research opportunities abound, but recruitment challenges restrict study entry due to organ impairment from advanced autoimmune disease or insurance denial of coverage for HCT. However, within several NIH sponsored trials there are ongoing immunologic, genomic and mechanistic studies to further understand the molecular mechanisms of autoimmunity, immune regulation and response to treatment. These clinical trials will provide basic scientists with insight into immunoregulatory pathways and clinicians with a context to weigh the progress and evidence in this evolving treatment for autoimmune diseases.
Copyright 2010 American Society for Blood and Marrow Transplantation. Published by Elsevier Inc. All rights reserved.
Figures
Figure 1
Both genetic and environmental factors play a role in the development of autoimmune disease. Development of autoimmune disease in adulthood suggests that multiple immunizing events are required to break immune tolerance. It is proposed that autologous HCT by “resetting” the immunological memory, may return the individual’s immune system to a pre-morbid state, resulting in a prolonged clinical remission.
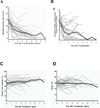
Figure 2
Improvements in serial Rodnan skin score (A), Health Assessment Questionnaire (B), Forced Vital Capacity, FVC (C) and Diffusion Capacity, DLCO (D) following immunoablation and autologous HCT. Gray solid lines depict individual patient parameters. Solid black lines are mean values and dashed lines represent the generalized estimating equation (GEE) for repeated measures. Mean Rodnan scores (0 is normal, 51 is maximal skin hardening throughout the body) decreased over time (p<0.001). Improvements in mean Health Assessment Scores (0 is normal) were also significant (p<0.001). The mean FVC values improved over time (p=0.01), while the mean DLCO did not change significantly (p=0.5). This research was originally published in Nash RA, McSweeney PA, Crofford LJ, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for severe systemic sclerosis: long-tern follow-up of the US multicenter pilot study. Blood 2007; 110:1386–96. Copyright the American Society of Hematology.
Similar articles
- Autologous hematopoietic stem cell transplantation for autoimmune disease--is it now ready for prime time?
Atkins HL, Muraro PA, van Laar JM, Pavletic SZ. Atkins HL, et al. Biol Blood Marrow Transplant. 2012 Jan;18(1 Suppl):S177-83. doi: 10.1016/j.bbmt.2011.11.020. Biol Blood Marrow Transplant. 2012. PMID: 22226104 Free PMC article. Review. - Stem cell transplantation for autoimmune diseases.
Gratwohl A, Passweg J, Gerber I, Tyndall A; International Stem Cell Project for Autoimmune Diseases. Gratwohl A, et al. Best Pract Res Clin Haematol. 2001 Dec;14(4):755-76. doi: 10.1053/beha.2001.0171. Best Pract Res Clin Haematol. 2001. PMID: 11924920 Review. - Stem Cell Therapy as a Treatment for Autoimmune Disease-Updates in Lupus, Scleroderma, and Multiple Sclerosis.
Ramalingam S, Shah A. Ramalingam S, et al. Curr Allergy Asthma Rep. 2021 Mar 24;21(3):22. doi: 10.1007/s11882-021-00996-y. Curr Allergy Asthma Rep. 2021. PMID: 33759038 Review. - Hematopoietic cell transplantation for benign hematological disorders and solid tumors.
Storb RF, Lucarelli G, McSweeney PA, Childs RW. Storb RF, et al. Hematology Am Soc Hematol Educ Program. 2003:372-97. doi: 10.1182/asheducation-2003.1.372. Hematology Am Soc Hematol Educ Program. 2003. PMID: 14633791 Review. - Stem cell transplantation for severe autoimmune diseases: progress and problems.
Marmont AM. Marmont AM. Haematologica. 1998 Aug;83(8):733-43. Haematologica. 1998. PMID: 9793258 Review.
Cited by
- Remission of refractory Crohn's disease after autologous hematopoietic stem cell transplantation.
Ruiz MA, Kaiser Junior RL, Gouvêa Faria MA, de Quadros LG. Ruiz MA, et al. Rev Bras Hematol Hemoter. 2015 Mar-Apr;37(2):136-9. doi: 10.1016/j.bjhh.2015.01.002. Epub 2015 Jan 30. Rev Bras Hematol Hemoter. 2015. PMID: 25818827 Free PMC article. No abstract available. - Allogeneic HSCT for Autoimmune Diseases: A Retrospective Study From the EBMT ADWP, IEWP, and PDWP Working Parties.
Greco R, Labopin M, Badoglio M, Veys P, Furtado Silva JM, Abinun M, Gualandi F, Bornhauser M, Ciceri F, Saccardi R, Lankester A, Alexander T, Gennery AR, Bader P, Farge D, Snowden JA. Greco R, et al. Front Immunol. 2019 Jul 4;10:1570. doi: 10.3389/fimmu.2019.01570. eCollection 2019. Front Immunol. 2019. PMID: 31333680 Free PMC article. Clinical Trial. - Autologous hematopoietic SCT normalizes miR-16, -155 and -142-3p expression in multiple sclerosis patients.
Arruda LC, Lorenzi JC, Sousa AP, Zanette DL, Palma PV, Panepucci RA, Brum DS, Barreira AA, Covas DT, Simões BP, Silva WA Jr, Oliveira MC, Malmegrim KC. Arruda LC, et al. Bone Marrow Transplant. 2015 Mar;50(3):380-9. doi: 10.1038/bmt.2014.277. Epub 2014 Dec 8. Bone Marrow Transplant. 2015. PMID: 25486582 - Stem cell therapy in autoimmune rheumatic diseases: a comprehensive review.
Liu B, Shu S, Kenny TP, Chang C, Leung PS. Liu B, et al. Clin Rev Allergy Immunol. 2014 Oct;47(2):244-57. doi: 10.1007/s12016-014-8445-8. Clin Rev Allergy Immunol. 2014. PMID: 25146442 Review. - Hematopoietic Stem Cells in Regenerative Medicine: Astray or on the Path?
Müller AM, Huppertz S, Henschler R. Müller AM, et al. Transfus Med Hemother. 2016 Jul;43(4):247-254. doi: 10.1159/000447748. Epub 2016 Jul 26. Transfus Med Hemother. 2016. PMID: 27721700 Free PMC article. Review.
References
- Nelson JL, Torrez R, Louie FM, Choe OS, Storb R, Sullivan KM. Pre-existing autoimmune disease in patients with longterm survival after allogeneic bone marrow transplantation. J Rheum. 1997;24(suppl 48):23–29. - PubMed
- Thomas ED. Pros and cons of stem cell transplantation for autoimmune disease. J Rheum. 1997;24(suppl 48):100–102. - PubMed
- Shlomchik MJ, Craft JE, Mamula MJ. From T to B and back again: positive feedback in systemic autoimmune disease. Nat Rev Immunol. 2001;1:147–153. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical