Chemokine profiling of Japanese encephalitis virus-infected mouse neuroblastoma cells by microarray and real-time RT-PCR: implication in neuropathogenesis - PubMed (original) (raw)
Chemokine profiling of Japanese encephalitis virus-infected mouse neuroblastoma cells by microarray and real-time RT-PCR: implication in neuropathogenesis
Nimesh Gupta et al. Virus Res. 2010 Jan.
Abstract
Japanese encephalitis (JE) is one of the leading causes of acute encephalopathy affecting children and adolescents in the tropics. JE virus (JEV) infection causes prominent neurological sequelae in approximately one-third of the survivors. In humans, the inflammatory response of CNS consequent to JEV induced viral encephalitis is mediated through chemokines released by various cells of CNS. In the present study, the chemokine profiles of mouse neuroblastoma cells (N2A) following JEV infection was analyzed by cDNA microarray followed by real-time RT-PCR. Eighty mRNA transcripts belonging to various functional classes exhibited significant alterations in gene expression. There was considerable induction of genes involved in apoptosis and anti-viral response. Modified levels of several transcripts involved in proinflammatory and anti-inflammatory processes exemplified the balance between opposing forces during JEV pathogenesis. Other genes displaying altered transcription included those associated with host translation, cellular metabolism, cell cycle, signal transduction, transcriptional regulation, protein trafficking, neurotransmitters, neuron maturation, protein modulators, ER stress and cytoskeletal proteins. The infection of neurons results in the synthesis of proinflammatory chemokines, which are early important mediators of leukocyte recruitment to sites of viral infection. Our results clearly suggest the implication of chemokines in neuropathogenesis of JEV infection leading to neurological sequelae. Pro- and anti-inflammatory agents targeted against chemokines such as CXCL10 may provide possible therapeutic modalities that can mitigate the morbidity associated with JEV infection of the CNS.
Figures
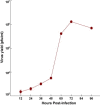
Fig. 1
Kinetics of JEV growth in Mouse N2A cells. Monolayers of N2A cells were infected with the JE S982 strain of JEV at an MOI of ∼5 and incubated at 37 °C. At various time intervals, samples were removed and virus titre was assayed. Values are mean ± SE of three replicates each.
Fig. 2
Morphological pattern of neuroblastoma cells infected with JEV. Monolayers of the mouse neuroblastoma cells were adsorbed with either live JE virus (multiplicity of infection (MOI) ∼5) or mock-infected for 1 h, at 37 °C. (a) N2A cells mock-infected at 36 hpi; (b) N2A cells infected with JEV at 36 hpi; (c) N2A cells infected with JEV at 72 hpi; (d) N2A cells infected with JEV at 96 hpi.
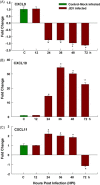
Fig. 3
Chemokine expression by JEV-infected mouse neuroblastoma. N2A cells were infected with JEV, at least three replicates per time point were collected at different time intervals, and analysis was done with real-time RT-PCR. The expression of (A) CXCL9, (B) CXCL10 and (C) CXCL11 are shown. Values are mean ± SE of three replicates each. *Significantly different from control at p < 0.05 by Dunnet's test.
Similar articles
- Viral Infection of the Central Nervous System and Neuroinflammation Precede Blood-Brain Barrier Disruption during Japanese Encephalitis Virus Infection.
Li F, Wang Y, Yu L, Cao S, Wang K, Yuan J, Wang C, Wang K, Cui M, Fu ZF. Li F, et al. J Virol. 2015 May;89(10):5602-14. doi: 10.1128/JVI.00143-15. Epub 2015 Mar 11. J Virol. 2015. PMID: 25762733 Free PMC article. - Innate immune mechanisms in Japanese encephalitis virus infection: effect on transcription of pattern recognition receptors in mouse neuronal cells and brain tissue.
Fadnis PR, Ravi V, Desai A, Turtle L, Solomon T. Fadnis PR, et al. Viral Immunol. 2013 Dec;26(6):366-77. doi: 10.1089/vim.2013.0016. Epub 2013 Nov 16. Viral Immunol. 2013. PMID: 24236856 Free PMC article. - Neuronal transcriptomic responses to Japanese encephalitis virus infection with a special focus on chemokine CXCL11 and pattern recognition receptors RIG-1 and MDA5.
Yu SP, Ong KC, Perera D, Wong KT. Yu SP, et al. Virology. 2019 Jan 15;527:107-115. doi: 10.1016/j.virol.2018.10.015. Epub 2018 Nov 24. Virology. 2019. PMID: 30481615 - Japanese encephalitis virus: Associated immune response and recent progress in vaccine development.
Kumar A, Sharma P, Shukla KK, Misra S, Nyati KK. Kumar A, et al. Microb Pathog. 2019 Nov;136:103678. doi: 10.1016/j.micpath.2019.103678. Epub 2019 Aug 19. Microb Pathog. 2019. PMID: 31437579 Review. - Unravelling the neuropathogenesis of Japanese encephalitis.
Myint KS, Gibbons RV, Perng GC, Solomon T. Myint KS, et al. Trans R Soc Trop Med Hyg. 2007 Oct;101(10):955-6. doi: 10.1016/j.trstmh.2007.04.004. Epub 2007 Jun 4. Trans R Soc Trop Med Hyg. 2007. PMID: 17544469 Review.
Cited by
- Japanese Encephalitis Virus-Infected Cells.
Sharma KB, Chhabra S, Kalia M. Sharma KB, et al. Subcell Biochem. 2023;106:251-281. doi: 10.1007/978-3-031-40086-5_10. Subcell Biochem. 2023. PMID: 38159231 - Gene expression analysis in the thalamus and cerebrum of horses experimentally infected with West Nile virus.
Bourgeois MA, Denslow ND, Seino KS, Barber DS, Long MT. Bourgeois MA, et al. PLoS One. 2011;6(10):e24371. doi: 10.1371/journal.pone.0024371. Epub 2011 Oct 4. PLoS One. 2011. PMID: 21991302 Free PMC article. - Upregulated expression of the antioxidant sestrin 2 identified by transcriptomic analysis of Japanese encephalitis virus-infected SH-SY5Y neuroblastoma cells.
Carr M, Gonzalez G, Martinelli A, Wastika CE, Ito K, Orba Y, Sasaki M, Hall WW, Sawa H. Carr M, et al. Virus Genes. 2019 Oct;55(5):630-642. doi: 10.1007/s11262-019-01683-x. Epub 2019 Jul 10. Virus Genes. 2019. PMID: 31292858 - Transcriptomic profile of host response in Japanese encephalitis virus infection.
Gupta N, Rao PV. Gupta N, et al. Virol J. 2011 Mar 4;8:92. doi: 10.1186/1743-422X-8-92. Virol J. 2011. PMID: 21371334 Free PMC article. - Respiratory syncytial virus prolifically infects N2a neuronal cells, leading to TLR4 and nucleolin protein modulations and RSV F protein co-localization with TLR4 and nucleolin.
Yuan X, Hu T, He H, Qiu H, Wu X, Chen J, Wang M, Chen C, Huang S. Yuan X, et al. J Biomed Sci. 2018 Feb 10;25(1):13. doi: 10.1186/s12929-018-0416-6. J Biomed Sci. 2018. PMID: 29427996 Free PMC article.
References
- Burke D.S., Monath T.P., Flaviviruses ., Knipe D.M., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., editors. 4th ed. vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2001. pp. 1043–1125. (Fields Virology).
- Chen J.P., Lu H.L., Lai S.L., Campanella G.S., Sung J.M., Lu M.Y., Wu-Hsieh B.A., Lin Y.L., Lane T.E., Luster A.D., Liao F. Dengue virus induces expression of CXC chemokine ligand 10/IFN-gamma-inducible protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J. Immunol. 2006;177:3185–3192. - PubMed
- Hsieh M.F., Lai S.L., Chen J.P., Sung J.M., Lin Y.L., Wu-Hsieh B.A., Gerard C., Luster A., Liao F. Both CXCR3 and CXCL10/IFN-inducible protein 10 are required for resistance to primary infection by dengue virus. J. Immunol. 2006;177:1855–1863. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources