Measured motion: searching for simplicity in spinal locomotor networks - PubMed (original) (raw)
Review
Measured motion: searching for simplicity in spinal locomotor networks
Sten Grillner et al. Curr Opin Neurobiol. 2009 Dec.
Abstract
Spinal interneurons are organized into networks that control the activity and output of the motor system. This review outlines recent progress in defining the rules that govern the assembly and function of spinal motor networks, focusing on three main areas. We first examine how subtle variations in the wiring diagrams and organization of locomotor networks in different vertebrates permits animals to adapt their motor programs to the demands of their physical environment. We discuss how the membrane properties of spinal interneurons, and their synaptic interactions, underlie the modulation of motor circuits and encoded motor behaviors. We also describe recent molecular genetic approaches to map and manipulate the connectivity and interactions of spinal interneurons and to assess the impact of such perturbations on network function and motor behavior.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
Figure 1. Amphibian metamorphosis is accompanied by a change in spinal locomotor pattern
(A) Tail-based swimming in pre-metamorphic (
stage 64) froglets. By this time, the tail has been resorbed and swimming is now produced by slower, bilaterally-synchronous cycles of hindlimb extension and flexion (upper panel). The isolated spinal cord/brain stem generates a fictive locomotor pattern in which left and right limb extensor motoneurons produce coincident bursts that alternate with bursts in left and right hindlimb flexor motoneurons. Adapted from ref. 16.
Figure 2. A model for the diversification of spinal motor neuron subtypes during vertebrate evolution
Lower branch. Inductive signaling along the dorsoventral axis of the neural tube involves the graded actions of sonic hedgehog (Shh) and Wnt proteins (not shown) secreted by the floor plate and adjacent ventral cells, and the patterned expression of homeodomain (HD) proteins by ventral progenitor cells and post-mitotic neurons. This dorso-ventral patterning pathway recruits expression of the LIM homeodomain proteins Lhx3 and Lhx4 by newly generated motor neurons, assigning a median motor column (MMC) – like identity. In primitive ancestral vertebrates such as the lamprey and hagfish the dominance of this inductive pathway confers all motor neurons with a MMC fate, providing the neural substrate for undulatory locomotor behaviors. Upper branch. Over the course of ∼500 million years of evolution, rostro-caudal inductive signals mediated by retinoids (RA) and fibroblast growth factors (FGF) establish the patterned expression of Hox homeodomain proteins in motor neuron progenitors and post-mitotic motor neurons. The engagement of Hox transcription factors, and an essential Hox accessory factor, FoxP1, drives motor neuron columnar and pool diversification, resulting in the formation of lateral motor column (LMC) neurons and their resident motor pools. This diversification process permits the spinal motor system to innervate the more complex set of peripheral motor targets that characterizes higher vertebrates and underlies intricate tetrapod gait patterns. The key intermediate step in this diversification process is presumed to involve the attenuation of non-canonical Wnt signaling, permitting the generation of motor neurons that lack Lhx3 and Lhx4 expression and progress to a hypaxial motor columnar (HMC) fate (not shown). This ground-state HMC-like column provides the neuronal substrate for the actions of the Hox/FoxP1 transcriptional program. For details, see text and ref 26.
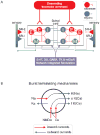
Fig. 3. Locomotor network of the lamprey
Diagrams show schematic representation of the brainstem and spinal components of the neural circuitry that generates rhythmic locomotor activity. A. All neuron symbols denote populations rather than single cells. Descending commands exerted through glutamatergic neurons excite all classes of spinal interneurons and motoneurons. The excitatory interneurons (E) excite all types of spinal neurons, i.e. the inhibitory glycinergic interneurons (I) that cross the midline to inhibit all neuron types on the contralateral side and motoneurons (M). Stretch receptor neurons are of two types; one excitatory (SR-E), which excites ipsilateral neurons and one inhibitory (SR-I), which crosses the midline to inhibit contralateral neurons and provide sensory feedback during actual swimming. In addition, metabotropic receptors are activated during locomotion and are an integral part of the network as indicated in the box below the network (5-HT, dopamine (DA), GABA, tachykinins (TK) and mGluRs). B. Cellular mechanisms contributing to burst termination. During a burst, Ca2+ ions entering via NMDA and voltage-dependent channels activate Ca-activated K+ channels (KCa) that will have a hyperpolarising effect with different time courses (fast (f) and slow (s)). Similarly the entry of Na+ ions (Na) during the burst will activate sodium-dependent K+ channels (KNa). Voltage-dependent K+ channels activated during the action potential are grouped and indicated as (KD).
Figure 4. Molecular programs of interneuron diversity in the ventral spinal cord
A. The position of origin of motor neurons (MN) and the four cardinal interneuron populations V0-V3) in the ventral spinal cord. B. Representative examples of interneuron classes derived from distinct ventral progenitor domains. Many of these interneuron subtypes can be distinguished by post-mitotic transcription factor expression, axonal trajectories and transmitter phenotype. C. Schematic diagram depicting proposed circuitry of distinct ventral interneuron classes, defined by transcriptional identity and physiological properties. Although many details of the link between molecular identity, network organization and physiological properties remain unclear, emerging evidence supports the view that molecular determinants of neuronal identity contribute to the assembly of spinal interneuron networks. For details, and references, see text.
Figure 5. Regulation of cellular and locomotor network properties by endogenous neuromodulators
In this schematic diagram, upward red arrow indicates enhanced activity, and downward blue arrow a reduction or depression in activity. Diverse modulatory effects include regulation of burst frequency, NMDA receptor dependent synaptic transmission, spike frequency adaptation, post-inhibitory rebound and phase-dependent presynaptic inhibition.
Similar articles
- Activity-dependent feedforward inhibition modulates synaptic transmission in a spinal locomotor network.
Parker D. Parker D. J Neurosci. 2003 Dec 3;23(35):11085-93. doi: 10.1523/JNEUROSCI.23-35-11085.2003. J Neurosci. 2003. PMID: 14657166 Free PMC article. - Reconfiguration of the spinal interneuronal network during locomotion in vertebrates.
Frigon A. Frigon A. J Neurophysiol. 2009 May;101(5):2201-3. doi: 10.1152/jn.00003.2009. Epub 2009 Mar 11. J Neurophysiol. 2009. PMID: 19279156 Review. - Continuous shifts in the active set of spinal interneurons during changes in locomotor speed.
McLean DL, Masino MA, Koh IY, Lindquist WB, Fetcho JR. McLean DL, et al. Nat Neurosci. 2008 Dec;11(12):1419-29. doi: 10.1038/nn.2225. Epub 2008 Nov 9. Nat Neurosci. 2008. PMID: 18997790 Free PMC article. - Assembly and function of spinal circuits for motor control.
Catela C, Shin MM, Dasen JS. Catela C, et al. Annu Rev Cell Dev Biol. 2015;31:669-98. doi: 10.1146/annurev-cellbio-100814-125155. Epub 2015 Sep 21. Annu Rev Cell Dev Biol. 2015. PMID: 26393773 Review. - Differential distribution of interneurons in the neural networks that control walking in the mudpuppy (Necturus maculatus) spinal cord.
Cheng J, Jovanovic K, Aoyagi Y, Bennett DJ, Han Y, Stein RB. Cheng J, et al. Exp Brain Res. 2002 Jul;145(2):190-8. doi: 10.1007/s00221-002-1102-0. Epub 2002 May 9. Exp Brain Res. 2002. PMID: 12110959
Cited by
- Mapping and cracking sensorimotor circuits in genetic model organisms.
Clark DA, Freifeld L, Clandinin TR. Clark DA, et al. Neuron. 2013 May 22;78(4):583-95. doi: 10.1016/j.neuron.2013.05.006. Neuron. 2013. PMID: 23719159 Free PMC article. - Multi-neuronal refractory period adapts centrally generated behaviour to reward.
Harris CA, Buckley CL, Nowotny T, Passaro PA, Seth AK, Kemenes G, O'Shea M. Harris CA, et al. PLoS One. 2012;7(7):e42493. doi: 10.1371/journal.pone.0042493. Epub 2012 Jul 31. PLoS One. 2012. PMID: 22860134 Free PMC article. - Generation of BAC transgenic tadpoles enabling live imaging of motoneurons by using the urotensin II-related peptide (ust2b) gene as a driver.
Bougerol M, Auradé F, Lambert FM, Le Ray D, Combes D, Thoby-Brisson M, Relaix F, Pollet N, Tostivint H. Bougerol M, et al. PLoS One. 2015 Feb 6;10(2):e0117370. doi: 10.1371/journal.pone.0117370. eCollection 2015. PLoS One. 2015. PMID: 25658845 Free PMC article. - Pattern of innervation and recruitment of different classes of motoneurons in adult zebrafish.
Ampatzis K, Song J, Ausborn J, El Manira A. Ampatzis K, et al. J Neurosci. 2013 Jun 26;33(26):10875-86. doi: 10.1523/JNEUROSCI.0896-13.2013. J Neurosci. 2013. PMID: 23804107 Free PMC article. - Evolution of patterning systems and circuit elements for locomotion.
Jung H, Dasen JS. Jung H, et al. Dev Cell. 2015 Feb 23;32(4):408-22. doi: 10.1016/j.devcel.2015.01.008. Dev Cell. 2015. PMID: 25710528 Free PMC article. Review.
References
- Grillner S. Biological pattern generation: The cellular and computational logic of networks in motion. Neuron. 2006;52:751–766. - PubMed
- Tunstall MJ, Roberts A, Soffe SR. Modelling inter-segmental coordination of neuronal oscillators: synaptic mechanisms for uni-directional coupling during swimming in Xenopus tadpoles. J Neurosci. 2002;13:143–158. - PubMed
- Grillner S, Kozlov A, Dario P, Stefanini C, Menciassi A, Lansner A, Hellgren Kotaleski J. Modeling a vertebrate motor system: pattern generation, steering and control of body orientation. Prog Brain Rev. 2007;165:221–234. - PubMed
- Kozlov A, Huss M, Lanner A, Hellgren Kotaleski J, Grillner S. Simple cellular and network control principles govern complex patterns of motor behaviour. Proc Natl Acad Sci USA. (in press). This modeling study employs large scale simulation of the locomotor system from the basal ganglia to the spinal networks with realistic number of neurons, to produce a motor pattern that resembles, in many features, that observed in vivo.
- Grillner S. The motor infrastructure: From ion channels to neuronal networks. Nature Reviews Neuroscience. 2003;4:573–586. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources