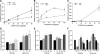Effects of 2,4-diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy - PubMed (original) (raw)
. 2010 Feb 1;19(3):454-67.
doi: 10.1093/hmg/ddp510. Epub 2009 Nov 6.
Jasbir Singh, Margrét Thorsteinsdóttir, Luciano Saieva, Elzbieta Slominski, John Thurmond, Thorkell Andrésson, Jun Zhang, Jonathan D Edwards, Louise R Simard, Livio Pellizzoni, Jill Jarecki, Arthur H M Burghes, Mark E Gurney
Affiliations
- PMID: 19897588
- PMCID: PMC2798721
- DOI: 10.1093/hmg/ddp510
Effects of 2,4-diaminoquinazoline derivatives on SMN expression and phenotype in a mouse model for spinal muscular atrophy
Matthew E R Butchbach et al. Hum Mol Genet. 2010.
Abstract
Proximal spinal muscular atrophy (SMA), one of the most common genetic causes of infant death, results from the selective loss of motor neurons in the spinal cord. SMA is a consequence of low levels of survival motor neuron (SMN) protein. In humans, the SMN gene is duplicated; SMA results from the loss of SMN1 but SMN2 remains intact. SMA severity is related to the copy number of SMN2. Compounds which increase the expression of SMN2 could, therefore, be potential therapeutics for SMA. Ultrahigh-throughput screening recently identified substituted quinazolines as potent SMN2 inducers. A series of C5-quinazoline derivatives were tested for their ability to increase SMN expression in vivo. Oral administration of three compounds (D152344, D153249 and D156844) to neonatal mice resulted in a dose-dependent increase in Smn promoter activity in the central nervous system. We then examined the effect of these compounds on the progression of disease in SMN lacking exon 7 (SMNDelta7) SMA mice. Oral administration of D156844 significantly increased the mean lifespan of SMNDelta7 SMA mice by approximately 21-30% when given prior to motor neuron loss. In summary, the C5-quinazoline derivative D156844 increases SMN expression in neonatal mouse neural tissues, delays motor neuron loss at PND11 and ameliorates the motor phenotype of SMNDelta7 SMA mice.
Figures
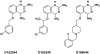
Figure 1.
The chemical structures of the three 2,4-diaminoquinazoline derivatives (D152344, D153249 and D156844) that were used in these experiments. These compounds are labeled as 5b, (S)-7a and 11a, respectively, in Ref. (42).
Figure 2.
Drug bioavailability and mSmn promoter activities in the CNS of neonatal SMNΔ7 carrier mice treated with 2,4-diaminoquinazoline derivatives. (A–C) Dose-dependent serum (dashed line with triangles) and brain (solid line with circles) levels of D152344 (A), D153249 (B) and D156844 (C) in neonatal SMNΔ7 carrier mice. (D–F) mSmn promoter-dependent β-galactosidase activity in the forebrains and spinal cords of SMNΔ7 carrier mice receiving differing doses of D152344 (D), D153249 (E) and D156844 (F). The β-galactosidase activity at each dose was normalized to the activity of vehicle-treated (0 mg/kg/day) samples. The doses administered for each compound are listed under each bar (n = 4/treatment dose). Key: *P ≤ 0.05 when compared with 0 mg/kg/day-treated mice.
Figure 3.
Oral administration of D156844 alters SMN gene expression in the spinal cord of SMNΔ7 SMA mice. (A) Changes in human-specific, full-length SMN (FL-SMN) mRNA levels in the spinal cord of mice treated with D156844 or vehicle for 5 days (n = 3/treatment group). (B) Changes in human-specific, SMNΔ7 mRNA levels in the spinal cord of mice treated with D156844 or vehicle for 5 days (n = 3/treatment group). (C) Representative immunoblot showing SMN and β-actin protein expression in spinal cord extracts from SMNΔ7 SMA mice treated with either D156844 or vehicle for 5 days. (D) Quantitation of SMN protein levels relative to β-actin protein in D156844- or vehicle-treated spinal cord extracts (n = 3/treatment group). SMN protein levels are expressed relative to those observed in age-matched carrier mice. (E) snRNP assembly activity in the spinal cord of SMNΔ7 SMA mice treated with either D156844 or vehicle for 5 days (n = 3/treatment group). snRNP assembly activity is measured by the amount of 32P-labeled U1 snRNA precipitated by antibodies against snRNP proteins (Sm proteins) as a consequence of SMN-dependent Sm core formation taking place in vitro using spinal cord extracts (50,69). snRNP assembly activity is expressed relative to that observed in age-matched carrier mice.
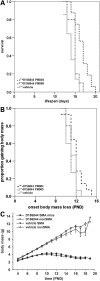
Figure 4.
Oral administration of D156844 improves the survival of and delays the onset of loss of body mass in neonatal SMNΔ7 SMA mice. (A) Kaplan–Meier survival plot for SMNΔ7 SMA mice receiving either vehicle (light grey solid line) or D156844 (3 mg/kg/day) beginning at either PND04 (black dashed line) or PND09 (grey dotted line). (B) Kaplan–Meier onset of body mass loss plot for SMNΔ7 SMA mice receiving either vehicle (light grey solid line) or D156844 (3 mg/kg/day) beginning at either PND04 (black dashed line) or PND09 (grey dotted line). (C) Body mass curves for SMNΔ7 SMA mice receiving either vehicle (triangles) or D156844 (3 mg/kg/day; circles) beginning at PND04.
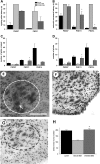
Figure 5.
Oral administration of D156844 improves the motor phenotype of SMNΔ7 SMA mice and reduces motor neuron loss in the lumbar spinal cord. (A) Righting reflex latencies at PND07 and PND11 for SMNΔ7 SMA mice treated with D156844 (3 mg/kg/day; dark grey bar) or vehicle (light grey bar) as compared with carrier (black bar) mice. (B) Vectorial movement latencies at PND07, PND11 and PND14 for SMNΔ7 SMA mice treated with D156844 or vehicle. (C) Spontaneous locomotor activity measured as the number of grids crossed in 1 min at PND07, PND11 and PND14 for SMNΔ7 SMA mice treated with D156844 or vehicle. (D) The number of pivots (90° turns) made in 1 min at PND07, PND11 and PND14 for SMNΔ7 SMA mice treated with D156844 or vehicle. For the phenotype data shown in (A–D), the sample sizes (n) for vehicle-, D156844-treated SMA mice and carrier mice at PND07 were 7, 5 and 5, respectively, at PND11, 5, 5 and 3, respectively and at PND14, 3, 5 and 3, respectively. Key for (A–D): *P < 0.05 when comparing vehicle-treated SMNΔ7 SMA mice to D156844-treated SMNΔ7 SMA mice. (E–G) Representative images of Cresyl violet-stained lumbar spinal cord sections from carrier mice (E) and SMNΔ7 SMA mice treated with vehicle (F) or D156844 (G) from PND04 until PND11. The dashed ovals represent the regions of interest in the ventral horn and arrow in (E) points to a motor neuron. Scale, 100 µm. (H) The number of motor neurons in the lumbar (L4-5) spinal cord of SMNΔ7 SMA mice (PND11) treated with D156844 or vehicle (n = 3/group). Key for (H): * P < 0.001 when comparing vehicle-treated SMNΔ7 SMA mice to D156844-treated SMNΔ7 SMA mice.
Figure 6.
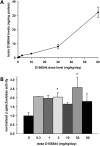
CNS bioavailability and mSmn promoter activity in prenatal mice receiving D156844. (A) D156844 levels in prenatal mouse brains whose dams were dosed with differing amounts of D156844 (0–60 mg/kg/day; n = 3/group) beginning at ED11.5. (B) β-Galactosidase activity—a marker for mSmn promoter activity—in the brains of mice whose dams received different doses of D156844 (0–60 mg/kg/day; n = 3/group) beginning at ED11.5. The β-galactosidase activity at each dose was normalized to the activity of vehicle-treated (0 mg/kg/day) samples. Key: *P ≤ 0.05 when compared with 0 mg/kg/day-treated mice.
Figure 7.
Prenatal administration of D156844 improves the survival and phenotype of SMNΔ7 SMA mice. (A) Kaplan–Meier survival plot for SMNΔ7 SMA mice receiving either vehicle (light grey solid line) or D156844 (3 mg/kg/day) beginning ED11.5 and continuing after birth (black dashed line) or ending at birth (grey dotted line). (B) Kaplan–Meier onset of body mass loss plot for SMNΔ7 SMA mice receiving either vehicle (light grey solid line) or D156844 [beginning ED11.5 and continuing after birth (black dashed line) or ending at birth (grey dotted line)]. (C) Body mass curves for SMNΔ7 SMA mice receiving either vehicle (triangles) or D156844 (3 mg/kg/day; circles) beginning at ED11.5.
Figure 8.
Therapeutic window of opportunity for protective effects of SMN2 induction by D156844.
Similar articles
- The DcpS inhibitor RG3039 improves motor function in SMA mice.
Van Meerbeke JP, Gibbs RM, Plasterer HL, Miao W, Feng Z, Lin MY, Rucki AA, Wee CD, Xia B, Sharma S, Jacques V, Li DK, Pellizzoni L, Rusche JR, Ko CP, Sumner CJ. Van Meerbeke JP, et al. Hum Mol Genet. 2013 Oct 15;22(20):4074-83. doi: 10.1093/hmg/ddt257. Epub 2013 May 31. Hum Mol Genet. 2013. PMID: 23727836 Free PMC article. - A SMN missense mutation complements SMN2 restoring snRNPs and rescuing SMA mice.
Workman E, Saieva L, Carrel TL, Crawford TO, Liu D, Lutz C, Beattie CE, Pellizzoni L, Burghes AH. Workman E, et al. Hum Mol Genet. 2009 Jun 15;18(12):2215-29. doi: 10.1093/hmg/ddp157. Epub 2009 Mar 27. Hum Mol Genet. 2009. PMID: 19329542 Free PMC article. - Protective effects of butyrate-based compounds on a mouse model for spinal muscular atrophy.
Butchbach MER, Lumpkin CJ, Harris AW, Saieva L, Edwards JD, Workman E, Simard LR, Pellizzoni L, Burghes AHM. Butchbach MER, et al. Exp Neurol. 2016 May;279:13-26. doi: 10.1016/j.expneurol.2016.02.009. Epub 2016 Feb 15. Exp Neurol. 2016. PMID: 26892876 Free PMC article. - Physical exercise training for type 3 spinal muscular atrophy.
Bartels B, Montes J, van der Pol WL, de Groot JF. Bartels B, et al. Cochrane Database Syst Rev. 2019 Mar 1;3(3):CD012120. doi: 10.1002/14651858.CD012120.pub2. Cochrane Database Syst Rev. 2019. PMID: 30821348 Free PMC article. Review. - Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
Cited by
- Evaluation of peripheral blood mononuclear cell processing and analysis for Survival Motor Neuron protein.
Kobayashi DT, Decker D, Zaworski P, Klott K, McGonigal J, Ghazal N, Sly L, Chung B, Vanderlugt J, Chen KS. Kobayashi DT, et al. PLoS One. 2012;7(11):e50763. doi: 10.1371/journal.pone.0050763. Epub 2012 Nov 30. PLoS One. 2012. PMID: 23226377 Free PMC article. - Spinal muscular atrophy: development and implementation of potential treatments.
Arnold WD, Burghes AH. Arnold WD, et al. Ann Neurol. 2013 Sep;74(3):348-62. doi: 10.1002/ana.23995. Ann Neurol. 2013. PMID: 23939659 Free PMC article. Review. - Mouse survival motor neuron alleles that mimic SMN2 splicing and are inducible rescue embryonic lethality early in development but not late.
Hammond SM, Gogliotti RG, Rao V, Beauvais A, Kothary R, DiDonato CJ. Hammond SM, et al. PLoS One. 2010 Dec 29;5(12):e15887. doi: 10.1371/journal.pone.0015887. PLoS One. 2010. PMID: 21249120 Free PMC article. - Assays for the identification and prioritization of drug candidates for spinal muscular atrophy.
Cherry JJ, Kobayashi DT, Lynes MM, Naryshkin NN, Tiziano FD, Zaworski PG, Rubin LL, Jarecki J. Cherry JJ, et al. Assay Drug Dev Technol. 2014 Aug;12(6):315-41. doi: 10.1089/adt.2014.587. Assay Drug Dev Technol. 2014. PMID: 25147906 Free PMC article. Review. - The effects of C5-substituted 2,4-diaminoquinazolines on selected transcript expression in spinal muscular atrophy cells.
Gentillon C, Connell AJ, Kirk RW, Butchbach MER. Gentillon C, et al. PLoS One. 2017 Jun 29;12(6):e0180657. doi: 10.1371/journal.pone.0180657. eCollection 2017. PLoS One. 2017. PMID: 28662219 Free PMC article.
References
- Crawford T.O., Pardo C.A. The neurobiology of childhood spinal muscular atrophy. Neurobiol. Dis. 1996;3:97–110. - PubMed
- Monani U.R., Lorson C.L., Parsons D.W., Prior T.W., Androphy E.J., Burghes A.H.M., McPherson J.D. A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Hum. Mol. Genet. 1999;8:1177–1183. - PubMed
- Lefebvre S., Bürglen L., Reboullet S., Clermont O., Burlet P., Viollet L., Benichou B., Cruaud C., Millasseau P., Zeviani M., et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. - PubMed
- Coovert D.D., Le T.T., McAndrew P.E., Strasswimmer J., Crawford T.O., Mendell J.R., Coulson S.E., Androphy E.J., Prior T.W., Burghes A.H.M. The survival motor neuron protein in spinal muscular atrophy. Hum. Mol. Genet. 1997;6:1205–1214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous