Targeting breast stem cells with the cancer preventive compounds curcumin and piperine - PubMed (original) (raw)
Comparative Study
Targeting breast stem cells with the cancer preventive compounds curcumin and piperine
Madhuri Kakarala et al. Breast Cancer Res Treat. 2010 Aug.
Abstract
The cancer stem cell hypothesis asserts that malignancies arise in tissue stem and/or progenitor cells through the dysregulation or acquisition of self-renewal. In order to determine whether the dietary polyphenols, curcumin, and piperine are able to modulate the self-renewal of normal and malignant breast stem cells, we examined the effects of these compounds on mammosphere formation, expression of the breast stem cell marker aldehyde dehydrogenase (ALDH), and Wnt signaling. Mammosphere formation assays were performed after curcumin, piperine, and control treatment in unsorted normal breast epithelial cells and normal stem and early progenitor cells, selected by ALDH positivity. Wnt signaling was examined using a Topflash assay. Both curcumin and piperine inhibited mammosphere formation, serial passaging, and percent of ALDH+ cells by 50% at 5 microM and completely at 10 microM concentration in normal and malignant breast cells. There was no effect on cellular differentiation. Wnt signaling was inhibited by both curcumin and piperine by 50% at 5 microM and completely at 10 microM. Curcumin and piperine separately, and in combination, inhibit breast stem cell self-renewal but do not cause toxicity to differentiated cells. These compounds could be potential cancer preventive agents. Mammosphere formation assays may be a quantifiable biomarker to assess cancer preventive agent efficacy and Wnt signaling assessment can be a mechanistic biomarker for use in human clinical trials.
Figures
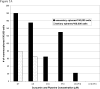
Figure 1
Figure 1A. Primary mammospheres formed from unsorted normal human breast epithelial cells in suspension culture for 10 days. Left picture incubation with curcumin 5 μM, right picture piperine 5 μM treament. Figure 1B Primary mammosphere number (±SEM) formed from unsorted normal human breast epithelial cells in suspension culture with curcumin (C5= curcumin 5 μM, C10= curcumin 10 μM); 1C piperine treatment (P5= piperine 5 μM, and P10= piperine 10μM), 1D curcumin plus piperine treatment
Figure 1
Figure 1A. Primary mammospheres formed from unsorted normal human breast epithelial cells in suspension culture for 10 days. Left picture incubation with curcumin 5 μM, right picture piperine 5 μM treament. Figure 1B Primary mammosphere number (±SEM) formed from unsorted normal human breast epithelial cells in suspension culture with curcumin (C5= curcumin 5 μM, C10= curcumin 10 μM); 1C piperine treatment (P5= piperine 5 μM, and P10= piperine 10μM), 1D curcumin plus piperine treatment
Figure 2
Figure 2A. Secondary and Tertiary sphere formation from human normal single epithelial cells in suspension with curcumin and piperine Figure 2B. Number of differentiated colonies formed from secondary sphere forming human breast stem cells with curcumin and piperine from time of plating versus treatment 48 hrs. after plating. Figure 2C. Immunostaining for CK14 myoepithelial marker in blue and CK18 luminal marker in red and mixed differentiated colonies formed in the presence of curcumin and piperine
Figure 2
Figure 2A. Secondary and Tertiary sphere formation from human normal single epithelial cells in suspension with curcumin and piperine Figure 2B. Number of differentiated colonies formed from secondary sphere forming human breast stem cells with curcumin and piperine from time of plating versus treatment 48 hrs. after plating. Figure 2C. Immunostaining for CK14 myoepithelial marker in blue and CK18 luminal marker in red and mixed differentiated colonies formed in the presence of curcumin and piperine
Figure 2
Figure 2A. Secondary and Tertiary sphere formation from human normal single epithelial cells in suspension with curcumin and piperine Figure 2B. Number of differentiated colonies formed from secondary sphere forming human breast stem cells with curcumin and piperine from time of plating versus treatment 48 hrs. after plating. Figure 2C. Immunostaining for CK14 myoepithelial marker in blue and CK18 luminal marker in red and mixed differentiated colonies formed in the presence of curcumin and piperine
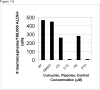
Figure 3
Figure 3A. Primary mammosphere number formed from ALDH+ presorted normal human breast stem/early progenitor cells in suspension culture with curcumin and piperine treatment (C5=curcumin 5 μM, c10=curcumin 10 μM, P5= piperine 5 μM, and P10=piperine 10 μM) compared to DMSO vehicle and no treatment (NT controls) Figure 3B Percent of ALDH1A1 positive cells after treatment of unsorted normal human breast epithelial cells with curcumin and piperine treatment
Figure 3
Figure 3A. Primary mammosphere number formed from ALDH+ presorted normal human breast stem/early progenitor cells in suspension culture with curcumin and piperine treatment (C5=curcumin 5 μM, c10=curcumin 10 μM, P5= piperine 5 μM, and P10=piperine 10 μM) compared to DMSO vehicle and no treatment (NT controls) Figure 3B Percent of ALDH1A1 positive cells after treatment of unsorted normal human breast epithelial cells with curcumin and piperine treatment
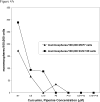
Figure 4
Figure 4A. Number of spheres formed from MCF7 or SUM 159 cells in suspension culture with 12 hrs. of curcumin and piperine treatment (C5=curcumin 5 μM;, C10=curcumin 10 μM; P5=piperine 5 μM; P10=piperine 10 μM; C5+P10=curcumin 5 μM and piperine 10 μM) Figure 4B Green fluorescence positivity in MCF7 Topflash assay with curcumin and piperine treatment
Figure 4
Figure 4A. Number of spheres formed from MCF7 or SUM 159 cells in suspension culture with 12 hrs. of curcumin and piperine treatment (C5=curcumin 5 μM;, C10=curcumin 10 μM; P5=piperine 5 μM; P10=piperine 10 μM; C5+P10=curcumin 5 μM and piperine 10 μM) Figure 4B Green fluorescence positivity in MCF7 Topflash assay with curcumin and piperine treatment
Similar articles
- Transcriptomic profiling of curcumin-treated human breast stem cells identifies a role for stearoyl-coa desaturase in breast cancer prevention.
Colacino JA, McDermott SP, Sartor MA, Wicha MS, Rozek LS. Colacino JA, et al. Breast Cancer Res Treat. 2016 Jul;158(1):29-41. doi: 10.1007/s10549-016-3854-4. Epub 2016 Jun 15. Breast Cancer Res Treat. 2016. PMID: 27306423 Free PMC article. - Implications of cancer stem cell theory for cancer chemoprevention by natural dietary compounds.
Li Y, Wicha MS, Schwartz SJ, Sun D. Li Y, et al. J Nutr Biochem. 2011 Sep;22(9):799-806. doi: 10.1016/j.jnutbio.2010.11.001. Epub 2011 Feb 4. J Nutr Biochem. 2011. PMID: 21295962 Free PMC article. Review. - Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells.
Li Y, Zhang T, Korkaya H, Liu S, Lee HF, Newman B, Yu Y, Clouthier SG, Schwartz SJ, Wicha MS, Sun D. Li Y, et al. Clin Cancer Res. 2010 May 1;16(9):2580-90. doi: 10.1158/1078-0432.CCR-09-2937. Epub 2010 Apr 13. Clin Cancer Res. 2010. PMID: 20388854 Free PMC article. - Synergistic effect of curcumin and piperine in suppression of DENA-induced hepatocellular carcinoma in rats.
Patial V, S M, Sharma S, Pratap K, Singh D, Padwad YS. Patial V, et al. Environ Toxicol Pharmacol. 2015 Sep;40(2):445-52. doi: 10.1016/j.etap.2015.07.012. Epub 2015 Jul 26. Environ Toxicol Pharmacol. 2015. PMID: 26278679 - Cancer stem cells: potential target for bioactive food components.
Kim YS, Farrar W, Colburn NH, Milner JA. Kim YS, et al. J Nutr Biochem. 2012 Jul;23(7):691-8. doi: 10.1016/j.jnutbio.2012.03.002. J Nutr Biochem. 2012. PMID: 22704055 Free PMC article. Review.
Cited by
- Progastrin Peptides Increase the Risk of Developing Colonic Tumors: Impact on Colonic Stem Cells.
Singh P, Sarkar S, Kantara C, Maxwell C. Singh P, et al. Curr Colorectal Cancer Rep. 2012 Dec;8(4):277-289. doi: 10.1007/s11888-012-0144-3. Curr Colorectal Cancer Rep. 2012. PMID: 23226720 Free PMC article. - Encapsulation of curcumin in diblock copolymer micelles for cancer therapy.
Alizadeh AM, Sadeghizadeh M, Najafi F, Ardestani SK, Erfani-Moghadam V, Khaniki M, Rezaei A, Zamani M, Khodayari S, Khodayari H, Mohagheghi MA. Alizadeh AM, et al. Biomed Res Int. 2015;2015:824746. doi: 10.1155/2015/824746. Epub 2015 Feb 22. Biomed Res Int. 2015. PMID: 25793208 Free PMC article. - Formulation of Piperine-Chitosan-Coated Liposomes: Characterization and In Vitro Cytotoxic Evaluation.
Imam SS, Alshehri S, Altamimi MA, Hussain A, Qamar W, Gilani SJ, Zafar A, Alruwaili NK, Alanazi S, Almutairy BK. Imam SS, et al. Molecules. 2021 May 29;26(11):3281. doi: 10.3390/molecules26113281. Molecules. 2021. PMID: 34072306 Free PMC article. - Dual-Enhanced Pluronic Nanoformulated Methotrexate-Based Treatment Approach for Breast Cancer: Development and Evaluation of In Vitro and In Vivo Efficiency.
Mansour A, Mahmoud MY, Bakr AF, Ghoniem MG, Adam FA, El-Sherbiny IM. Mansour A, et al. Pharmaceutics. 2022 Nov 30;14(12):2668. doi: 10.3390/pharmaceutics14122668. Pharmaceutics. 2022. PMID: 36559161 Free PMC article. - Nutritional Metabolomics in Diet-Breast Cancer Relations: Current Research, Challenges, and Future Directions-A Review.
Vahid F, Hajizadeghan K, Khodabakhshi A. Vahid F, et al. Biomedicines. 2023 Jun 27;11(7):1845. doi: 10.3390/biomedicines11071845. Biomedicines. 2023. PMID: 37509485 Free PMC article. Review.
References
- Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev. 2004;14:43–7. - PubMed
- Lim E, Vaillant F, Wu D, Forrest NC, Pal B, Hart AH, Asselin-Labat ML, Gyorki DE, Ward T, Partanen A, Feleppa F, Huschtscha LI, Thorne HJ, Fox SB, Yan M, French JD, Brown MA, Smyth GK, Visvader JE, Lindeman GJ. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–13. - PubMed
- Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG, Liu S, Schott A, Hayes D, Birnbaum D, Wicha MS, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–67. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical