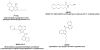Discriminative stimulus functions of methanandamide and delta(9)-THC in rats: tests with aminoalkylindoles (WIN55,212-2 and AM678) and ethanol - PubMed (original) (raw)
Discriminative stimulus functions of methanandamide and delta(9)-THC in rats: tests with aminoalkylindoles (WIN55,212-2 and AM678) and ethanol
Torbjörn U C Järbe et al. Psychopharmacology (Berl). 2010 Jan.
Abstract
Objective: The aim of the study was to characterize in vivo the aminoalkylindoles WIN55,212-2 (WIN) and AM678 (naphthalen-1-yl(1-pentyl-1H-indol-3-yl)methanone) as cannabinoid receptor (CB(1)R) ligands using drug discrimination. Tests also involved delta(9)-tetrahydrocannabinol (THC) and R-(+)-methanandamide (mAEA), a metabolically stable analog of the endogenous ligand anandamide, as well as the CB(1)R selective antagonist/inverse agonist rimonabant; tests with ethanol assessed pharmacological specificity. We used two different drug discriminations (mAEA and THC) allowing us to explore potential differences in CB(1)R activation which could be attributed to variations in their respective CB(1)R signaling mechanisms.
Methods: There were two concurrently trained groups of rats. One group discriminated between i.p. injected vehicle and 10 mg/kg mAEA. The other group was trained to discriminate between vehicle and 1.8 mg/kg THC.
Results: Dose generalization curves for AM678, WIN55,212-2, THC, and mAEA suggested the following rank order of potency: AM678 > WIN55,212-2 > or = THC > mAEA in both drug discrimination groups. Challenge by 1 mg/kg rimonabant resulted in shifts to the right of the generalization curves for the two aminoalkylindoles (4.4-fold for AM678 and 11.3-fold for WIN in the mAEA group, whereas for the THC group, the corresponding values were 13 and 2.6, respectively), suggesting surmountable antagonism. Ethanol did not generalize in either of the two groups, suggesting pharmacological specificity.
Conclusion: Data are congruent with the general observation that there is substantial overlap in the discriminative stimulus effects of CB(1)R ligands across different chemical classes. However, the quantitative differences in the interactions between the two aminoalkylindoles and rimonabant in the two discrimination groups suggest subtle variations in the ligand-receptor activation(s).
Figures
Fig. 1
Chemical structures of cannabinergic agonists used in study
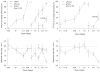
Fig. 2
Generalization test results (top) and corresponding response rate data (bottom) for mAEA (_R_-(+)-methanandamide; _n_=10–11), Δ9-THC (_n_=10–20) as well as for ETOH (ethanol; _n_=9–18) in the mAEA (10 mg/kg)- versus vehicle-trained rats (left), and Δ9-THC (_n_=12) as well as for ETOH (ethanol; _n_=9–19) in the Δ9-THC (1.8 mg/kg)-versus vehicle-trained rats (right); sessions began 20 min after i.p. administration. The generalization results represent the mean (± SEM) percentage of lever presses on the drug (mAEA, top left; Δ9-THC, top right) appropriate lever out of the total number of lever presses emitted during a test session (_Y_-axis); doses examined in milligrams per kilogram (_X_-axis). Rate refers to the mean (± SEM) number of lever presses per second emitted during a test session (_Y_-axis); doses in milligrams per kilogram (_X_-axis). Dotted lines represent the ±95% confidence limits of vehicle control response rate determined from the initial six reinforcement cycles of the vehicle training sessions immediately preceding these tests; symbols outside the confidence limits are considered significantly different from control. Data points are based on one to two observations for each rat and were obtained on separate test days. Numbers within brackets indicate the number of rats responding (i.e., accumulating at least ten responses on either lever and thus obtaining at least one reinforcement) out of the total number used for the test. Test results are based on sessions of a maximum of six reinforcements (12 food pellets) or 20 min, whichever occurred first. V vehicle
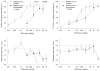
Fig. 3
Generalization test results (top) and the corresponding response rate data (bottom) for WIN55,212-2 alone (open hexagons) and when combined with 1 mg/kg rimonabant (filled hexagons), 30 min after i.p. administration. Test results for the 10-mg/kg mAEA-versus vehicle-trained rats are shown in the two left panels (_open hexagons; n_=9–19; _filled hexagons; n_=8–18 except for the highest test dose where _n_=7), and the results for the 1.8-mg/kg Δ9-THC-versus vehicle-trained rats are depicted in the two right panels (_open hexagons: n_=9–21; _filled hexagons: n_=9–19). Drug lever responding results represent the mean (± SEM) percentage of lever presses on the drug (mAEA, top left; Δ9-THC, top right) appropriate lever out of the total number of lever presses emitted during a test session (_Y_-axis); doses examined in milligrams per kilogram (_X_-axis). Rate refers to the mean (± SEM) number of lever presses per second emitted during a test session (_Y_-axis); doses in milligrams per kilogram (X-axis). Other details are as described in the legend for Fig. 2
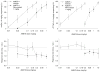
Fig. 4
Generalization test results (top) for AM678 alone (open triangles) and when combined with 1 mg/kg rimonabant (filled triangles) and the corresponding response rate data (bottom), 30 min after i.p. administration. Test results for the 10-mg/kg mAEA- versus vehicle-trained rats are shown in the two left panels (_open triangles; n_=8–18; _filled triangles; n_=8–10) and the results for the 1.8-mg/kg Δ9-THC- versus vehicle-trained rats are depicted in the two right panels (_open triangles; n_=20–21; _filled triangles; n_=10–21). Drug lever responding results represent the mean (± SEM) percentage of lever presses on the drug (mAEA, top left; Δ9-THC, top right) appropriate lever out of the total number of lever presses emitted during a test session (_Y_-axis); doses examined in milligrams per kilogram (_X_-axis). Rate refers to the mean (± SEM) number of lever presses per second emitted during a test session (_Y_-axis); doses in milligrams per kilogram (_X_-axis). Other details are as described in the legend for Fig. 2
Similar articles
- Discriminative stimulus functions in rats of AM1346, a high-affinity CB1R selective anandamide analog.
Järbe TU, Li C, Liu Q, Makriyannis A. Järbe TU, et al. Psychopharmacology (Berl). 2009 Apr;203(2):229-39. doi: 10.1007/s00213-008-1199-3. Epub 2008 Jun 3. Psychopharmacology (Berl). 2009. PMID: 18521574 Free PMC article. - Antagonism of discriminative stimulus effects of delta(9)-THC and (R)-methanandamide in rats.
Järbe TU, Liu Q, Makriyannis A. Järbe TU, et al. Psychopharmacology (Berl). 2006 Jan;184(1):36-45. doi: 10.1007/s00213-005-0225-y. Epub 2005 Nov 24. Psychopharmacology (Berl). 2006. PMID: 16307294 - Discriminative stimulus functions of AM-1346, a CB1R selective anandamide analog in rats trained with Delta9-THC or (R)-methanandamide (AM-356).
Järbe TU, Lamb RJ, Liu Q, Makriyannis A. Järbe TU, et al. Psychopharmacology (Berl). 2006 Oct;188(3):315-23. doi: 10.1007/s00213-006-0517-x. Epub 2006 Sep 5. Psychopharmacology (Berl). 2006. PMID: 16953384 - Differentiation between low- and high-efficacy CB1 receptor agonists using a drug discrimination protocol for rats.
Järbe TU, LeMay BJ, Halikhedkar A, Wood J, Vadivel SK, Zvonok A, Makriyannis A. Järbe TU, et al. Psychopharmacology (Berl). 2014 Feb;231(3):489-500. doi: 10.1007/s00213-013-3257-8. Epub 2013 Sep 5. Psychopharmacology (Berl). 2014. PMID: 24005529 Free PMC article. - [Drug discrimination properties and cytotoxicity of the cannabinoid receptor ligands].
Tomiyama K, Funada M. Tomiyama K, et al. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2012 Jun;47(3):135-43. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2012. PMID: 22894054 Review. Japanese.
Cited by
- A critical assessment of the abuse, dependence and associated safety risks of naturally occurring and synthetic cannabinoids.
Heal DJ, Gosden J, Smith SL. Heal DJ, et al. Front Psychiatry. 2024 Jun 10;15:1322434. doi: 10.3389/fpsyt.2024.1322434. eCollection 2024. Front Psychiatry. 2024. PMID: 38915848 Free PMC article. Review. - Molecular and Behavioral Pharmacological Characterization of Abused Synthetic Cannabinoids MMB- and MDMB-FUBINACA, MN-18, NNEI, CUMYL-PICA, and 5-Fluoro-CUMYL-PICA.
Gamage TF, Farquhar CE, Lefever TW, Marusich JA, Kevin RC, McGregor IS, Wiley JL, Thomas BF. Gamage TF, et al. J Pharmacol Exp Ther. 2018 May;365(2):437-446. doi: 10.1124/jpet.117.246983. Epub 2018 Mar 16. J Pharmacol Exp Ther. 2018. PMID: 29549157 Free PMC article. - Baths salts, spice, and related designer drugs: the science behind the headlines.
Baumann MH, Solis E Jr, Watterson LR, Marusich JA, Fantegrossi WE, Wiley JL. Baumann MH, et al. J Neurosci. 2014 Nov 12;34(46):15150-8. doi: 10.1523/JNEUROSCI.3223-14.2014. J Neurosci. 2014. PMID: 25392483 Free PMC article. Review. - JWH-018 in rhesus monkeys: differential antagonism of discriminative stimulus, rate-decreasing, and hypothermic effects.
Rodriguez JS, McMahon LR. Rodriguez JS, et al. Eur J Pharmacol. 2014 Oct 5;740:151-9. doi: 10.1016/j.ejphar.2014.06.023. Epub 2014 Jun 24. Eur J Pharmacol. 2014. PMID: 24972243 Free PMC article. - Antagonism of ∆⁹-THC induced behavioral effects by rimonabant: time course studies in rats.
Järbe TU, Gifford RS, Makriyannis A. Järbe TU, et al. Eur J Pharmacol. 2010 Dec 1;648(1-3):133-8. doi: 10.1016/j.ejphar.2010.09.006. Epub 2010 Sep 18. Eur J Pharmacol. 2010. PMID: 20854804 Free PMC article.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, Makriyannis A. (R)-Methanandamide: a chiral novel anandamide possessing higher potency and metabolic stability. J Med Chem. 1994;37:1889–1893. - PubMed
- Alici T, Appel JB. Increasing the selectivity of the discriminative stimulus effects of Δ9-tetrahydrocannabinol: complete generalization with methanandamide. Pharmacol Biochem Behav. 2004;79:431–437. - PubMed
- Basavarajappa BS, Hungund BL. Role of the endocannabinoid system in the development of tolerance to alcohol. Alcohol Alcohol. 2005;40:15–24. - PubMed
- Bergman J, France CP, Holtzman SG, Katz JL, Koek W, Stephens DN. Agonist efficacy, drug dependence, and medications development: preclinical evaluation of opioid, dopaminergic, and GABAA-ergic ligands. Psychopharmacology (Berl) 2000;153:67–84. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 9158/PHS HHS/United States
- K02 DA000253/DA/NIDA NIH HHS/United States
- 7215/PHS HHS/United States
- DA 03801/DA/NIDA NIH HHS/United States
- 13429/PHS HHS/United States
- R37 DA003801/DA/NIDA NIH HHS/United States
- R01 DA009064/DA/NIDA NIH HHS/United States
- 00253/PHS HHS/United States
- 00152/PHS HHS/United States
- R01 DA003801/DA/NIDA NIH HHS/United States
- DA09064/DA/NIDA NIH HHS/United States
- P01 DA009158/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous