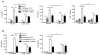Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury - PubMed (original) (raw)
Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury
Zubin M Bamboat et al. Hepatology. 2010 Feb.
Abstract
Endogenous ligands such as high-mobility group box 1 (HMGB1) and nucleic acids are released by dying cells and bind Toll-like receptors (TLRs). Because TLR9 sits at the interface of microbial and sterile inflammation by detecting both bacterial and endogenous DNA, we investigated its role in a model of segmental liver ischemia-reperfusion (I/R) injury. Mice were subjected to 1 hour of ischemia and 12 hours of reperfusion before assessment of liver injury, cytokines, and reactive oxygen species (ROS). Wild-type (WT) mice treated with an inhibitory cytosine-guanosine dinucleotide (iCpG) sequence and TLR9(-/-) mice had markedly reduced serum alanine aminotransferase (ALT) and inflammatory cytokines after liver I/R. Liver damage was mediated by bone marrow-derived cells because WT mice transplanted with TLR9(-/-) bone marrow were protected from hepatic I/R injury. Injury in WT mice partly depended on TLR9 signaling in neutrophils, which enhanced production of ROS, interleukin-6 (IL-6), and tumor necrosis factor (TNF). In vitro, DNA released from necrotic hepatocytes increased liver nonparenchymal cell (NPC) and neutrophil cytokine secretion through a TLR9-dependent mechanism. Inhibition of both TLR9 and HMGB1 caused maximal inflammatory cytokine suppression in neutrophil cultures and conferred even greater protection from I/R injury in vivo.
Conclusion: TLR9 serves as an endogenous sensor of tissue necrosis that exacerbates the innate immune response during liver I/R. Combined blockade of TLR9 and HMGB1 represents a clinically relevant, novel approach to limiting I/R injury.
Conflict of interest statement
Conflict of interest. The authors have declared that no conflict of interest exists.
Figures
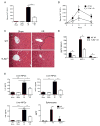
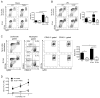
Figure 1. TLR9−/− mice have less injury after liver I/R
WT and TLR9−/− mice were subjected to liver I/R or sham procedure and serum ALT was measured (A) 12 h later or (B) at serial time points. (C) Liver sections are shown from the ischemic lobes of WT and TLR9−/− mice at 12 h (magnification x200). Non-viable tissue is shown by dashed lines. Liver sections are representative of 4 independent experiments (5 mice/group). (D) Serum cytokines were determined in WT and TLR9−/− mice after 12 h of I/R. (E) Ischemic liver CD45+ NPCs or bulk splenocytes from WT and TLR9−/− mice after 12 h of I/R or sham procedure were cultured in media without restimulation. Supernatant cytokine levels were measured 24 h later. Cytokines were undetectable in splenocyte cultures from mice undergoing sham procedure (unpublished data). Data in A, B, D and E represent means ± SEM and are representative of at least 3 independent experiments (5 mice/group). *p<0.05; **p<0.01; ***p<0.001.
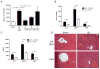
Figure 2. TLR9 inhibition protects WT mice from liver I/R injury
WT mice received 100 μg iCpG s.c. immediately before, or 6 or 8 h following the onset of I/R or 100 μg control oligodeoxynucleotide (Ctrl ODN) sequence just prior to I/R. (A) Serum ALT and (B) serum cytokines were measured at 12 h. (C) Ischemic liver CD45+ NPCs from WT mice treated with Ctrl ODN or iCpG just prior to initiation of ischemia as in (B), were cultured in media after 12 h of I/R. Supernatant levels of cytokines were determined 24 h later. (D) Representative liver H&E staining at 12 h is shown (magnification x200). Non-viable patches of ischemic lobes are demarcated by dashed lines. Data in (A–C) represent means ± SEM and in (A–D) are representative of at least 3 independent experiments (5 mice/group). *p<0.05.
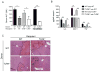
Figure 3. TLR9 in bone marrow derived cells mediates liver injury after I/R
Bone marrow chimeras were generated using WT (CD45.1 or CD45.2) and TLR9−/− (CD45.2) mice. Non-irradiated WT and TLR9−/− mice served as controls (non-chimeras). 12 h after I/R, (A) serum ALT and (B) serum cytokines were measured. (C) H&E-stained sections of ischemic liver lobes from TLR9−/− chimeric mice 12 h after I/R (magnification x100). Non-viable patches are demarcated by dashed lines. Data in (A) and (B) represent means ± SEM and in (A–C) are representative of 3 independent experiments (5 mice/group). *p< 0.05; **p<0.01.
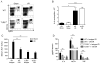
Figure 4. TLR9 is necessary for neutrophil-mediated I/R injury
Neutrophil (CD11bhiLy6G+) recruitment to the ischemic liver after 12 h of I/R or sham surgery is represented as (A) percentage of hepatic CD45+ leukocytes or (B) absolute number of neutrophils. WT or TLR9−/− mice were depleted of neutrophils and (C) serum ALT and (D) serum cytokines were determined 12 h after I/R. Data in (B–D) represent means ± SEM and in (A–D) are representative of at least 2 independent experiments (4–6 mice/group). *p<0.05; **p<0.01; ***p<0.001.
Figure 5. Liver I/R increases neutrophil oxidative burst via TLR9
CD45+ NPCs from the ischemic livers of WT and TLR9−/− mice 12 h after (A) sham laparotomy or (B) I/R were isolated. Contour plots depict neutrophils as a percentage of hepatic leukocytes in the presence or absence of E.coli stimulation in vitro. Bar graphs depict mean ROS production ± SEM by WT and TLR9−/− neutrophils pooled from 3 separate experiments, each with similar results. (C) 4 × 106 WT (CD45.1+) neutrophils were injected into TLR9−/−(CD45.2+) recipients just prior to I/R. 12 h later, ischemic liver CD45+ NPCs were isolated and then assessed for the presence of donor and recipient neutrophils. The bar graph depicts mean ROS production ± SEM by donor and recipient neutrophils pooled from 2 separate experiments, each with similar results. (D) I/R was performed in TLR9−/− mice following intrasplenic injection of varying numbers of WT or TLR9−/− neutrophils (PMNs). Serum ALT was measured 12 h later. Data in (D) are representative of 3 independent experiments. (A-D) were performed with (4–6 mice/group). *p<0.05; **p<0.01.
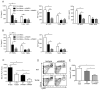
Figure 6. Liver NPCs are activated by endogenous hepatocyte DNA through TLR9
WT and TLR9−/− (A) CD45+ NPCs or (B) neutrophils were cultured in media alone or with conditioned (Con) media from 106 or 5 × 106 necrotic hepatocytes. Some wells containing conditioned media (from 5 × 106 necrotic hepatocytes) were pre-treated with DNAse I for 2 h prior to co-culture with NPCs or neutrophils. Supernatant cytokines were measured 24 (NPCs) or 12 h (neutrophils) later. Levels of MCP-1 were undetectable for neutrophil cultures in (B) (unpublished data). Data represent means ± SEM and are representative of 3 independent experiments. *p<0.05; **p<0.01.
Figure 7. Absence of TLR9 combined with HMGB1 blockade increases hepatic resistance to I/R injury
WT and TLR9−/− (A) CD45+ NPCs or (B) neutrophils were cultured in conditioned (Con) media from necrotic hepatocytes and αHMGB1. Certain wells containing αHMGB1 were pre-treated with DNAse I for 2 h. After 12 h or 24 h, supernatant cytokines were measured. (C) WT and TLR9−/− mice were injected with αHMGB1 or isotype control 1 h prior to I/R. Serum ALT was measured 12 h later. (D) The percentage of neutrophils in the ischemic livers of mice subjected to 12 h of I/R following treatment with αHMGB1 or isotype is shown. (E) WT mice were pre-treated with iCpG, αHMGB1 or both just prior to I/R. Serum ALT was measured 12 h later. Data represent means ± SEM and are representative of 3 (A, B) or 2 (C, D, E) independent experiments (4–6 mice/group). *p<0.05; **p<0.01.
References
- Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology. 2000;32:169–73. - PubMed
- Jaeschke H, Lemasters JJ. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–57. - PubMed
- Lentsch AB, Yoshidome H, Kato A, Warner RL, Cheadle WG, Ward PA, Edwards MJ. Requirement for interleukin-12 in the pathogenesis of warm hepatic ischemia/reperfusion injury in mice. Hepatology. 1999;30:1448–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources