Dicer1 functions as a haploinsufficient tumor suppressor - PubMed (original) (raw)
Dicer1 functions as a haploinsufficient tumor suppressor
Madhu S Kumar et al. Genes Dev. 2009.
Abstract
While the global down-regulation of microRNAs (miRNAs) is a common feature of human tumors, its genetic basis is largely undefined. To explore this question, we analyzed the consequences of conditional Dicer1 mutation (Dicer1 "floxed" or Dicer1(fl)) on several mouse models of cancer. Here we show Dicer1 functions as a haploinsufficient tumor suppressor gene. Deletion of a single copy of Dicer1 in tumors from Dicer1(fl/+) animals led to reduced survival compared with controls. These tumors exhibited impaired miRNA processing but failed to lose the wild-type Dicer1 allele. Moreover, tumors from Dicer1(fl/fl) animals always maintained one functional Dicer1 allele. Consistent with selection against full loss of Dicer1 expression, enforced Dicer1 deletion caused inhibition of tumorigenesis. Analysis of human cancer genome copy number data reveals frequent deletion of DICER1. Importantly, however, the gene has not been reported to undergo homozygous deletion, suggesting that DICER1 is haploinsufficient in human cancer. These findings suggest Dicer1 may be an important haploinsufficient tumor suppressor gene and, furthermore, that other factors controlling miRNA biogenesis may also function in this manner.
Figures
Figure 1.
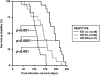
Dicer1 mutation reduces post-infection survival in a genetically engineered mouse model of K-Rasdriven lung cancer. KrasLSL-G12D mice, either wild type or heterozygous or homozygous conditional for Dicer1 (KD+/+, KDfl/+, and KDfl/fl, respectively), were intranasally infected with Ad-Cre, and survival was assessed. Median survival was 194 d for KD+/+ mice, 108 d for KDfl/+ mice, and 143 d for KDfl/fl mice. Statistical significance was assessed by the log-rank test.
Figure 2.
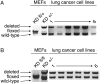
Dicer1 undergoes hemizygous loss in lung tumors. (A) DNA was prepared from KrasLSL-G12D mouse embryonic fibroblasts (MEFs) either heterozygous conditional or mutant for Dicer1 (KDfl/+ and KD+/−) and lung cancer cell lines from KPDfl/+ mice, and the Dicer1 locus was examined by PCR. (B) DNA was prepared from KrasLSL-G12D mouse embryonic fibroblasts either homozygous conditional or heterozygous mutant for Dicer1 (KDfl/fl and KD+/−) and lung cancer cell lines from KPDfl/fl mice, and the Dicer1 locus was examined by PCR.
Figure 3.
Hemizygous deletion of Dicer1 causes a global reduction in steady-state miRNA levels. (A) miRNA profiling and hierarchical clustering were performed on lung cancer cell lines either wild type (KPD+/+) or heterozygous for Dicer1 (KPD+/− and KPDfl/−). (B) Small RNA Northern blotting analysis of miRNAs and glutamine tRNA was performed on lung cancer cell lines wild type or heterozygous for Dicer1 as above.
Figure 4.
Complete deletion of Dicer1 inhibits sarcoma development. (A) KPDfl/+ and KPDfl/− sarcoma cell lines were infected with MSCV.CreERT2.puro. Cre-mediated recombination was induced by treatment with 4-OHT for defined time points. DNA was prepared, and recombination of the Dicer1 locus was assessed by PCR. (B) Two independent KPDfl/− sarcoma cell lines infected with MSCV.CreERT2.puro were injected subcutaneously into C57Bl6/129SV F1 animals. Animals were treated with or without tamoxifen by intraperitoneal injection, and tumor growth was measured over time. Values are mean ± SEM (n = 8 each). (C) Tumors were isolated from KPDfl/− sarcoma cell line transplants treated with or without tamoxifen. DNA was prepared, and recombination of the Dicer1 locus was assessed by PCR. Asterisk corresponds to the wild-type Dicer1 locus from the C57Bl6/129SV F1 host animals.
Comment in
- Journal club. A cancer geneticist looks at the link between small RNA molecules and cancer.
Marine JC. Marine JC. Nature. 2010 Apr 29;464(7293):1249. doi: 10.1038/4641249e. Nature. 2010. PMID: 20428124 No abstract available.
Similar articles
- Stop the dicing in hematopoiesis: what have we learned?
Alemdehy MF, Erkeland SJ. Alemdehy MF, et al. Cell Cycle. 2012 Aug 1;11(15):2799-807. doi: 10.4161/cc.21077. Epub 2012 Aug 1. Cell Cycle. 2012. PMID: 22801545 Free PMC article. - Unique haploinsufficient role of the microRNA-processing molecule Dicer1 in a murine colitis-associated tumorigenesis model.
Yoshikawa T, Otsuka M, Kishikawa T, Takata A, Ohno M, Shibata C, Kang YJ, Yoshida H, Koike K. Yoshikawa T, et al. PLoS One. 2013 Sep 2;8(9):e71969. doi: 10.1371/journal.pone.0071969. eCollection 2013. PLoS One. 2013. PMID: 24023722 Free PMC article. - Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo.
Lambertz I, Nittner D, Mestdagh P, Denecker G, Vandesompele J, Dyer MA, Marine JC. Lambertz I, et al. Cell Death Differ. 2010 Apr;17(4):633-41. doi: 10.1038/cdd.2009.202. Epub 2009 Dec 18. Cell Death Differ. 2010. PMID: 20019750 Free PMC article. - The role of Dicer1 in the male reproductive tract.
Björkgren I, Sipilä P. Björkgren I, et al. Asian J Androl. 2015 Sep-Oct;17(5):737-41. doi: 10.4103/1008-682X.155542. Asian J Androl. 2015. PMID: 25994652 Free PMC article. Review. - An update on the central nervous system manifestations of DICER1 syndrome.
de Kock L, Priest JR, Foulkes WD, Alexandrescu S. de Kock L, et al. Acta Neuropathol. 2020 Apr;139(4):689-701. doi: 10.1007/s00401-019-01997-y. Epub 2019 Apr 5. Acta Neuropathol. 2020. PMID: 30953130 Review.
Cited by
- Interrelationship between microsatellite instability and microRNA in gastrointestinal cancer.
Yamamoto H, Adachi Y, Taniguchi H, Kunimoto H, Nosho K, Suzuki H, Shinomura Y. Yamamoto H, et al. World J Gastroenterol. 2012 Jun 14;18(22):2745-55. doi: 10.3748/wjg.v18.i22.2745. World J Gastroenterol. 2012. PMID: 22719182 Free PMC article. Review. - Integrated transcriptional and competitive endogenous RNA networks are cross-regulated in permissive molecular environments.
Ala U, Karreth FA, Bosia C, Pagnani A, Taulli R, Léopold V, Tay Y, Provero P, Zecchina R, Pandolfi PP. Ala U, et al. Proc Natl Acad Sci U S A. 2013 Apr 30;110(18):7154-9. doi: 10.1073/pnas.1222509110. Epub 2013 Mar 27. Proc Natl Acad Sci U S A. 2013. PMID: 23536298 Free PMC article. - Global microRNA elevation by inducible Exportin 5 regulates cell cycle entry.
Iwasaki YW, Kiga K, Kayo H, Fukuda-Yuzawa Y, Weise J, Inada T, Tomita M, Ishihama Y, Fukao T. Iwasaki YW, et al. RNA. 2013 Apr;19(4):490-7. doi: 10.1261/rna.036608.112. Epub 2013 Feb 19. RNA. 2013. PMID: 23431327 Free PMC article. - Synthetic lethality between Rb, p53 and Dicer or miR-17-92 in retinal progenitors suppresses retinoblastoma formation.
Nittner D, Lambertz I, Clermont F, Mestdagh P, Köhler C, Nielsen SJ, Jochemsen A, Speleman F, Vandesompele J, Dyer MA, Schramm A, Schulte JH, Marine JC. Nittner D, et al. Nat Cell Biol. 2012 Sep;14(9):958-65. doi: 10.1038/ncb2556. Epub 2012 Aug 5. Nat Cell Biol. 2012. PMID: 22864477 - Modeling of miRNA and drug action in the EGFR signaling pathway.
Li J, Pandey V, Kessler T, Lehrach H, Wierling C. Li J, et al. PLoS One. 2012;7(1):e30140. doi: 10.1371/journal.pone.0030140. Epub 2012 Jan 11. PLoS One. 2012. PMID: 22253908 Free PMC article.
References
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. - PubMed
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. - PubMed
- Esquela-Kerscher A, Trang P, Wiggins JF, Patrawala L, Cheng A, Ford L, Weidhaas JB, Brown D, Bader AG, Slack FJ. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle. 2008;7:759–764. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials