Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects - PubMed (original) (raw)
Optimized peptide vaccines eliciting extensive CD8 T-cell responses with therapeutic antitumor effects
Hyun-Il Cho et al. Cancer Res. 2009.
Abstract
A major challenge for developing effective therapeutic vaccines against cancer is overcoming immunologic tolerance to tumor-associated antigens that are expressed on both malignant cells and normal tissues. Herein, we describe a novel vaccination approach, TriVax, that uses synthetic peptides representing CD8 T-cell epitopes, Toll-like receptor agonists that function as potent immunologic adjuvants and costimulatory anti-CD40 antibodies to generate large numbers of high-avidity antigen-reactive T cells capable of recognizing and killing tumor cells. Our results show that TriVax induced huge numbers of long-lasting antigen-specific CD8 T cells that displayed significant antitumor effects in vivo. The administration of a TriVax formulation containing a CD8 T-cell epitope derived from a melanosomal antigen (Trp2(180-188)) elicited antigen-specific CD8 T cells that induced systemic autoimmunity (vitiligo). More important, TriVax immunization was effective in eliciting potent protective antitumor immunity as well as remarkable therapeutic effects against established B16 melanoma. This therapeutic effect was mediated by CD8 T cells via perforin-mediated lysis and required the participation of type-I IFN but not IFNgamma. These results suggest that similar strategies would be applicable for the design of effective vaccination for conducting clinical studies in cancer patients.
Conflict of interest statement
Conflict of interest: The authors state that there is no financial interest in this work.
Figures
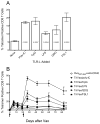
Figure 1. Effect of TLR-Ls on peptide vaccination with anit-CD40 mAb
(A), B6 mince (3 per group) were immunized i.v. with Ova 257–264 peptide and anti-CD40 mAb and one of the following TLR-L: poly-IC (TLR3-L), CpG (TLR9-L), Lps (TLR4-L), GDQ (TLR7-L), FSL1 (TLR6/2-L). Seven days later, antigen-specific CD8 T cell responses were measured in blood by using tetramer analysis. Results represent the means and SD for each group where p values compared to the no TLR group are shown inside of each bar. (B), The mice received a second identical immunization (on day 21) and the frequency of tetramer-specific CD8 T cells in peripheral blood was followed in individual mice throughout various time points. Results correspond to the man and SD for each group. Arrows, times of vaccination. P values were calculated using two-way ANOVA test (*p < 0.0001 for TriVax versus peptide+anti-CD40 mAb).
Figure 2. Immunization with Trp2180-–88-TriVax induces strong anti-tumor Cd8 T cell responses
B6mice were vaccinated i.v. on days 0 and 14 with Trp2180–188-TriVax/poly-IC.(A), On days 7 and 22, blood samples were evaluated by tetramer analysis. Each point represents the value for each individual mouse and horizontal line represents the average value of the group. (B), Eight days after the boost, CD8 T cells were purified from pooled splenocytes and antigen-induced IFNγ and TNFα secretion was evaluated by EliSpot. (C), Cytolytic activity of freshly isolated CD8 T cells, assessed by a 51Cr-release assay. (D), B6 mice (8 per group) were immunized on days −24 and −12 or only on day −12 with Trp2180–188-TriVax/poly-IC. Twelve days after the last immunization, the mice received 3 × 105 B16F10 melanoma cells i.v. Survival is compared against unvaccinated group (No Vax) or group that received Ova55–63-TriVax/poly-IC (negative control). Kaplan–Mayer survival curves for all groups of mice are shown. P values were determined by log-rank tests as compared to the negative control group.
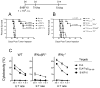
Figure 3. Therapeutic anti-tumor effects of Trp2180–188-TriVax
(A), B6 mice (4 per group) received 3 × 105 B16Fl0 cells i.v., and 3 and 11 days later the mice were vaccinated with Trp2180–188-TriVax or Ova55–63-TriVax. A non-vaccinated group (No Vax) was also included. On Day 24 (when the control mice appeared sick), the presence of B16 pulmonary nodules was evaluated. Results are presented as “Numbers of Lung Tumors” and “Lung Weights” for individual mice. Representative photographs of lungs of 2 mice from each group are shown. Splenocytes from mice of the 2 vaccinated groups in “A” were evaluated for Trp2-specific CD8 T cells by tetramer analysis (B), and antigen-induced IFNγ production in EliSpot assays (C). P values were calculated using unpaired Student’s test.
Figure 4. Soluble peptide is more effective than peptide emulsified in IFA in TriVal
(A), B6 mice (4 per group) received 3 × 105 B16F10 cells i.v. and 3 and 11 days later were vaccinated either i.v. or s.c. with Trp2180–188-TriVax or Ova55–63-TriVax. Peptides were emulsified in IFA for the s.c. vaccines. A non-vaccinated group (No Vax) was included. On day 24 all the mice were the presence of B16 pulmonary tumors was evaluated as described in Figure 3A. Splenocytes from individual mice were evaluated for Trp2-specific CD8 T cells by tetramer analysis (B) or EliSpot assaya (C) P values were calculated using unpaired Student’s test.
Figure 5. Therapeutic TriVax results in increased survival
(A), B6 mice (8 per group) received 3 different doses of B16F10 cells i.v. (as indicated) and 3 days later the mice were immunized with either Trp2180–188-TriVax, Ova55–63-TriVax or left unvaccinated (No Vax). P values were determined by log-rank tests. (B), At the termination of the experiment presented in (A), survivor mice were pooled, randomized and received s.c. tumor challenge with either B16F10 or B16F10-kb− cells (5 × 105 cell/mouse). Naï ve, unvaccinated mice inoculated with the same number of tumore cells were included as control. Tumore sizes were determined in individual mice by measurements of 2 opposing diameters and are presented as tumore areas in mm2. Each data point corresponds to the means and SD for each group of mice (5 per group). P value was calculated using two-ways ANOVA test.
Figure 6. Effector mechanisms involved in anti−tumor effects of TriVax
(A), B6 mice (8 per group) received with 1 × 10 5 B16F10 cells i.v. Various subsets of immune effector cells (CD8 T cells, CD4 T cells or NK cells) were depleted using mAb on days −3 and −1 before receiving the TriVax injection. An addition mAb injection was administered 2 days after vaccinatioin. TriVax immunizations were administered on days 3 and 14 after tumor injections. Kaplan–Mayer survival curves for all groups of mice are shown. P values were determined by log-rank tests. (B), Therapeutic effects of Trp2180–188-TriVax in WT B6 mice (WT), IFNγ−/−, IFNαβR−/−, and Prf−/− mice were evaluated a described above. Results were evaluated for statistical significance using log-rank test. (C), In a parallel experiment freshly isolated spleen CD8 T cells from WT, IFNγ−/−, IFNαβR−/−, mice were tested for cytolytic activity in a 5-h 51Cr-release assay as described in Figure 2C.
Similar articles
- In vivo expansion, persistence, and function of peptide vaccine-induced CD8 T cells occur independently of CD4 T cells.
Assudani D, Cho HI, DeVito N, Bradley N, Celis E. Assudani D, et al. Cancer Res. 2008 Dec 1;68(23):9892-9. doi: 10.1158/0008-5472.CAN-08-3134. Cancer Res. 2008. PMID: 19047170 Free PMC article. - Improved tumor immunity using anti-tyrosinase related protein-1 monoclonal antibody combined with DNA vaccines in murine melanoma.
Saenger YM, Li Y, Chiou KC, Chan B, Rizzuto G, Terzulli SL, Merghoub T, Houghton AN, Wolchok JD. Saenger YM, et al. Cancer Res. 2008 Dec 1;68(23):9884-91. doi: 10.1158/0008-5472.CAN-08-2233. Cancer Res. 2008. PMID: 19047169 Free PMC article. - T Cells Modified with CD70 as an Alternative Cellular Vaccine for Antitumor Immunity.
Lee SE, Shin AR, Sohn HJ, Cho HI, Kim TG. Lee SE, et al. Cancer Res Treat. 2020 Jul;52(3):747-763. doi: 10.4143/crt.2019.721. Epub 2020 Feb 14. Cancer Res Treat. 2020. PMID: 32065848 Free PMC article. - Improving T cell responses to modified peptides in tumor vaccines.
Buhrman JD, Slansky JE. Buhrman JD, et al. Immunol Res. 2013 Mar;55(1-3):34-47. doi: 10.1007/s12026-012-8348-9. Immunol Res. 2013. PMID: 22936035 Free PMC article. Review. - The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination?
Slingluff CL Jr. Slingluff CL Jr. Cancer J. 2011 Sep-Oct;17(5):343-50. doi: 10.1097/PPO.0b013e318233e5b2. Cancer J. 2011. PMID: 21952285 Free PMC article. Review.
Cited by
- Cancer associated aberrant protein O-glycosylation can modify antigen processing and immune response.
Madsen CB, Petersen C, Lavrsen K, Harndahl M, Buus S, Clausen H, Pedersen AE, Wandall HH. Madsen CB, et al. PLoS One. 2012;7(11):e50139. doi: 10.1371/journal.pone.0050139. Epub 2012 Nov 26. PLoS One. 2012. PMID: 23189185 Free PMC article. - Specific medicinal plant polysaccharides effectively enhance the potency of a DC-based vaccine against mouse mammary tumor metastasis.
Chang WT, Lai TH, Chyan YJ, Yin SY, Chen YH, Wei WC, Yang NS. Chang WT, et al. PLoS One. 2015 Mar 31;10(3):e0122374. doi: 10.1371/journal.pone.0122374. eCollection 2015. PLoS One. 2015. PMID: 25825910 Free PMC article. - A systematic review on poly(I:C) and poly-ICLC in glioblastoma: adjuvants coordinating the unlocking of immunotherapy.
De Waele J, Verhezen T, van der Heijden S, Berneman ZN, Peeters M, Lardon F, Wouters A, Smits ELJM. De Waele J, et al. J Exp Clin Cancer Res. 2021 Jun 25;40(1):213. doi: 10.1186/s13046-021-02017-2. J Exp Clin Cancer Res. 2021. PMID: 34172082 Free PMC article. Review. - Enhancing Dendritic Cell-based Immunotherapy with IL-2/Monoclonal Antibody Complexes for Control of Established Tumors.
Kim MT, Richer MJ, Gross BP, Norian LA, Badovinac VP, Harty JT. Kim MT, et al. J Immunol. 2015 Nov 1;195(9):4537-44. doi: 10.4049/jimmunol.1501071. Epub 2015 Sep 25. J Immunol. 2015. PMID: 26408669 Free PMC article. - Triple peptide vaccination as consolidation treatment in women affected by ovarian and breast cancer: Clinical and immunological data of a phase I/II clinical trial.
Antonilli M, Rahimi H, Visconti V, Napoletano C, Ruscito I, Zizzari IG, Caponnetto S, Barchiesi G, Iadarola R, Pierelli L, Rughetti A, Bellati F, Panici PB, Nuti M. Antonilli M, et al. Int J Oncol. 2016 Apr;48(4):1369-78. doi: 10.3892/ijo.2016.3386. Epub 2016 Feb 8. Int J Oncol. 2016. PMID: 26892612 Free PMC article. Clinical Trial.
References
- Cavallo F, Offringa R, van der Burg SH, Forni G, Melief CJ. Vaccination for treatment and prevention of cancer in animal models. Adv Immunol. 2006;90:175–213. - PubMed
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol. 2009;27:83–117. - PubMed
- Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–15. - PubMed
- Van Der Bruggen P, Zhang Y, Chaux P, et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol Rev. 2002;188:51–64. - PubMed
- Groothuis T, Neefjes J. The ins and outs of intracellular peptides and antigen presentation by MHC class I molecules. Curr Top Microbiol Immunol. 2005;300:127–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01CA103921/CA/NCI NIH HHS/United States
- R01 CA080782/CA/NCI NIH HHS/United States
- R01CA136828/CA/NCI NIH HHS/United States
- R01 CA080782-09/CA/NCI NIH HHS/United States
- R01 CA136828-01A2/CA/NCI NIH HHS/United States
- R01 CA136828/CA/NCI NIH HHS/United States
- R01 CA103921-07/CA/NCI NIH HHS/United States
- R01 CA103921/CA/NCI NIH HHS/United States
- R01CA80782/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials