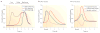A new perspective on the role of the orbitofrontal cortex in adaptive behaviour - PubMed (original) (raw)
Review
. 2009 Dec;10(12):885-92.
doi: 10.1038/nrn2753. Epub 2009 Nov 11.
Affiliations
- PMID: 19904278
- PMCID: PMC2835299
- DOI: 10.1038/nrn2753
Review
A new perspective on the role of the orbitofrontal cortex in adaptive behaviour
Geoffrey Schoenbaum et al. Nat Rev Neurosci. 2009 Dec.
Abstract
The orbitofrontal cortex (OFC) is crucial for changing established behaviour in the face of unexpected outcomes. This function has been attributed to the role of the OFC in response inhibition or to the idea that the OFC is a rapidly flexible associative-learning area. However, recent data contradict these accounts, and instead suggest that the OFC is crucial for signalling outcome expectancies. We suggest that this function--signalling of expected outcomes--can also explain the crucial role of the OFC in changing behaviour in the face of unexpected outcomes.
Figures
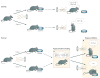
Figure 1. Showing orbitofrontal involvement in flexible, adaptive behaviour in rats using an odour discrimination reversal task
The events in a simple odour discrimination reversal task that demonstrates the involvement of the orbitofrontal cortex in flexible, adaptive behaviour. Rats sample an odour from a port. After sampling, they decide whether to respond at a nearby fluid well. One odour predicts a tasty sucrose solution, whereas a second odour predicts an unpleasant-tasting quinine solution. As illustrated in the top panel, rats rapidly learn to discriminate between the odours, drinking from the fluid well after sampling the sucrose-predicting odour but withholding that response after sampling the quinine-predicting odour. After this has been learned, the odour–outcome associations can be reversed, such that the sucrose-predicting odour comes to predict quinine and vice versa. This is illustrated in the bottom panel. Initially rats respond on the basis of what they have learned previously. However, normal rats rapidly recode these associations (a) and inhibit their old response patterns in favour of new ones (b); rats with damage to the orbitofrontal cortex fail to change their behaviour as rapidly.
Figure 2. Stylized plots of reward-responsive neurons in the orbitofrontal cortex
a | In discrimination learning tasks, neurons in the rat orbitofrontal cortex (OFC) initially fire in response to either the rewarding reinforcer (for example, sucrose) or the aversive reinforcer (for example, quinine) (dark blue line). After a number of trials the neurons also start to fire in anticipation of the reinforcer (red line). Finally, they also come to fire in response to cues that predict the reinforcer (purple line). b | These OFC neurons do not stop firing in response to the reward, even after many trials. Moreover, their firing is not stronger in response to an unexpected reward or weaker in response to the omission of a reward left panel). In this respect, OFC neurons are unlike dopamine (DA) neurons in the ventral tegmental area (VTA), which are also reward responsive but which fire more strongly in response to unexpected rewards and decrease firing when an expected reward is not delivered (right panel). Based on data from REFS –.
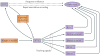
Figure 3. The proposed role of the orbitofrontal cortex in flexible, adaptive behaviour
A summary of several proposals reviewed in the text for explaining the role of the orbitofrontal cortex (OFC) in facilitating reversal learning. At the top is the historical proposal that output from the OFC directly inhibits ‘pre-potent’ responses and is therefore crucial for reversal learning. Below is the more recent idea that the OFC functions as a highly flexible associative reference table to directly guide correct responding and so is vital for reversal learning. At the bottom is our alternative proposal that the OFC is crucial for reversal learning because it drives associative learning in other structures. According to this proposal, the OFC does this by supporting the generation of teaching signals (for example, by the ventral tegmental area (VTA)) when actual outcomes do not match signals from the OFC regarding expected outcomes. BLA, basolateral amygdala; mPFC, medial prefrontal cortex.
Similar articles
- Primate orbitofrontal cortex and adaptive behaviour.
Roberts AC. Roberts AC. Trends Cogn Sci. 2006 Feb;10(2):83-90. doi: 10.1016/j.tics.2005.12.002. Epub 2005 Dec 27. Trends Cogn Sci. 2006. PMID: 16380289 - The essential role of primate orbitofrontal cortex in conflict-induced executive control adjustment.
Mansouri FA, Buckley MJ, Tanaka K. Mansouri FA, et al. J Neurosci. 2014 Aug 13;34(33):11016-31. doi: 10.1523/JNEUROSCI.1637-14.2014. J Neurosci. 2014. PMID: 25122901 Free PMC article. - Reward stability determines the contribution of orbitofrontal cortex to adaptive behavior.
Riceberg JS, Shapiro ML. Riceberg JS, et al. J Neurosci. 2012 Nov 14;32(46):16402-9. doi: 10.1523/JNEUROSCI.0776-12.2012. J Neurosci. 2012. PMID: 23152622 Free PMC article. - Orbitofrontal cortex, associative learning, and expectancies.
Schoenbaum G, Roesch M. Schoenbaum G, et al. Neuron. 2005 Sep 1;47(5):633-6. doi: 10.1016/j.neuron.2005.07.018. Neuron. 2005. PMID: 16129393 Free PMC article. Review. - Taking stock of value in the orbitofrontal cortex.
Knudsen EB, Wallis JD. Knudsen EB, et al. Nat Rev Neurosci. 2022 Jul;23(7):428-438. doi: 10.1038/s41583-022-00589-2. Epub 2022 Apr 25. Nat Rev Neurosci. 2022. PMID: 35468999 Free PMC article. Review.
Cited by
- A new framework for cortico-striatal plasticity: behavioural theory meets in vitro data at the reinforcement-action interface.
Gurney KN, Humphries MD, Redgrave P. Gurney KN, et al. PLoS Biol. 2015 Jan 6;13(1):e1002034. doi: 10.1371/journal.pbio.1002034. eCollection 2015 Jan. PLoS Biol. 2015. PMID: 25562526 Free PMC article. - Region specific activation of the AKT and mTORC1 pathway in response to excessive alcohol intake in rodents.
Laguesse S, Morisot N, Phamluong K, Ron D. Laguesse S, et al. Addict Biol. 2017 Nov;22(6):1856-1869. doi: 10.1111/adb.12464. Epub 2016 Oct 20. Addict Biol. 2017. PMID: 27766766 Free PMC article. - Change detection, multiple controllers, and dynamic environments: insights from the brain.
Pearson JM, Platt ML. Pearson JM, et al. J Exp Anal Behav. 2013 Jan;99(1):74-84. doi: 10.1002/jeab.5. Epub 2012 Dec 5. J Exp Anal Behav. 2013. PMID: 23344989 Free PMC article. - Value and choice as separable and stable representations in orbitofrontal cortex.
Kimmel DL, Elsayed GF, Cunningham JP, Newsome WT. Kimmel DL, et al. Nat Commun. 2020 Jul 10;11(1):3466. doi: 10.1038/s41467-020-17058-y. Nat Commun. 2020. PMID: 32651373 Free PMC article. - μ Opioid Antagonist Naltrexone Partially Abolishes the Antidepressant Placebo Effect and Reduces Orbitofrontal Cortex Encoding of Reinforcement.
Peciña M, Chen J, Lyew T, Karp JF, Dombrovski AY. Peciña M, et al. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021 Oct;6(10):1002-1012. doi: 10.1016/j.bpsc.2021.02.009. Epub 2021 Mar 6. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021. PMID: 33684624 Free PMC article. Clinical Trial.
References
- Harlow JM. Recovery after passage of an iron bar through the head. Publ Massachusetts Med Soc. 1868;2:329–346.
- Damasio H, Grabowski T, Frank R, Galaburda AM, Damasio AR. The return of Phineas Gage: clues about the brain from the skull of a famous patient. Science. 1994;264:1102–1105. - PubMed
- Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Comp Neurol. 1995;7:1–24. - PubMed
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol. 1972;36:362–377. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 MH080865-01A1/MH/NIMH NIH HHS/United States
- R01 MH080865/MH/NIMH NIH HHS/United States
- R01 AG027097/AG/NIA NIH HHS/United States
- R01 DA015718/DA/NIDA NIH HHS/United States
- R01 AG027097-03/AG/NIA NIH HHS/United States
- R01 DA015718-06A2/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources