Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy - PubMed (original) (raw)
Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy
Shaolin Yang et al. Psychiatry Res. 2009.
Abstract
Previous studies have shown significantly lower metabolism and functional activity in the anterior cingulate cortex (ACC) of human cocaine addicts. The present study examined whether this ACC hypoactivity is associated with altered glutamate (Glu), the primary excitatory neurotransmitter in the central nervous system (CNS), which has been recently implicated in drug addiction. Participants comprised 14 chronic cocaine addicts and 14 matched healthy volunteers who were examined using (1)H magnetic resonance spectroscopy at 3 T. A new quantification strategy for echo time (TE)-averaged point-resolved spectroscopy (PRESS) was applied to disentangle relaxation effects from J-evolution of coupled spin systems such as Glu. The concentrations of Glu as well as N-acetyl aspartate (NAA), total creatine (tCr), choline-containing compounds (tCho), and myo-inositol (Ins) were estimated from both groups. Glu/tCr was significantly lower in chronic cocaine users compared to control subjects and was significantly correlated with years of cocaine use. Glu/tCr was also positively correlated with NAA/tCr. NAA/tCr significantly decreased with age but was not significantly different between the two groups. These findings suggest a metabolic/neurotransmitter dysregulation associated with cocaine addiction and support a possible therapeutic intervention strategy aimed at normalizing the Glu transmission and function in the treatment of cocaine addiction.
Figures
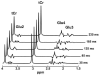
Fig. 1
Simulated PRESS spectra of tCr and Glu at multiple TEs (TE = 35 ms, 85 ms, 135 ms, 185 ms, and 235 ms, respectively), in which relaxation was not considered in the simulation. The singlet resonances of tCr at 3.02 ppm and 3.92 ppm remain unchanged through different TEs. In contrast, due to _J_-evolution of coupled spins, the C4 proton resonance of Glu at 2.35 ppm (Glu4), the C3 proton resonance of Glu around 2.08 ppm (Glu3), and the C2 proton resonance of Glu at 3.75 ppm (Glu2) demonstrate complex variation with TE.
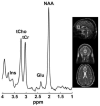
Fig. 2
TE-averaged PRESS spectrum acquired from a single voxel (white box) encompassing the rACC of a healthy volunteer (NAA = _N_-acetyl aspartate, tCr = total creatine, tCho = choline-containing compounds, Glu = glutamate, and Ins = _myo_-inositol).
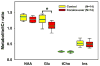
Fig. 3
Metabolite levels (in ratio to tCr) in the rACC of chronic cocaine users (red) and healthy controls (yellow) (NAA = _N_-acetyl aspartate, tCr = total creatine, tCho = choline-containing compounds, Glu = glutamate, and Ins = _myo_-inositol). Glu/tCr is significantly lower (15.88%) (F1,25=10.2; p < 0.005) in the cocaine-user group compared to the control group, after correcting for age. The thick light-green lines mark the mean values and the thin black lines mark the median values. Bars indicate the 5th/95th percentiles.
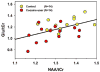
Fig. 4
Significant positive correlation between Glu/tCr and NAA/tCr (Glu = glutamate, NAA = _N_-acetyl aspartate, and tCr = total creatine) (partial R = 0.503; p < 0.01), accounting for age, in the rACC of chronic cocaine users (red) and healthy controls (yellow).
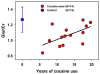
Fig. 5
Significant positive correlation between Glu/tCr and years of cocaine use (Glu = glutamate and tCr = total creatine) (partial R = 0.579; p<0.05), accounting for age and current cocaine use, in the rACC of chronic cocaine users (red). The mean and standard deviation of Glu/tCr in the control group (blue) are also shown for comparison.
Similar articles
- Glutamatergic and neurometabolic alterations in chronic cocaine users measured with (1) H-magnetic resonance spectroscopy.
Hulka LM, Scheidegger M, Vonmoos M, Preller KH, Baumgartner MR, Herdener M, Seifritz E, Henning A, Quednow BB. Hulka LM, et al. Addict Biol. 2016 Jan;21(1):205-17. doi: 10.1111/adb.12217. Epub 2014 Dec 30. Addict Biol. 2016. PMID: 25600822 - Psychostimulant drug effects on glutamate, Glx, and creatine in the anterior cingulate cortex and subjective response in healthy humans.
White TL, Monnig MA, Walsh EG, Nitenson AZ, Harris AD, Cohen RA, Porges EC, Woods AJ, Lamb DG, Boyd CA, Fekir S. White TL, et al. Neuropsychopharmacology. 2018 Jun;43(7):1498-1509. doi: 10.1038/s41386-018-0027-7. Epub 2018 Mar 6. Neuropsychopharmacology. 2018. PMID: 29511334 Free PMC article. Clinical Trial. - Anterior cingulate and cerebellar GABA and Glu correlations measured by ¹H J-difference spectroscopy.
Waddell KW, Zanjanipour P, Pradhan S, Xu L, Welch EB, Joers JM, Martin PR, Avison MJ, Gore JC. Waddell KW, et al. Magn Reson Imaging. 2011 Jan;29(1):19-24. doi: 10.1016/j.mri.2010.07.005. Epub 2010 Sep 29. Magn Reson Imaging. 2011. PMID: 20884148 Free PMC article. - Spectroscopic meta-analyses reveal novel metabolite profiles across methamphetamine and cocaine substance use disorder.
Smucny J, Maddock RJ. Smucny J, et al. Drug Alcohol Depend. 2023 Jul 1;248:109900. doi: 10.1016/j.drugalcdep.2023.109900. Epub 2023 Apr 26. Drug Alcohol Depend. 2023. PMID: 37148676 Free PMC article. Review. - Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings.
Yildiz-Yesiloglu A, Ankerst DP. Yildiz-Yesiloglu A, et al. Prog Neuropsychopharmacol Biol Psychiatry. 2006 Aug 30;30(6):969-95. doi: 10.1016/j.pnpbp.2006.03.012. Epub 2006 May 4. Prog Neuropsychopharmacol Biol Psychiatry. 2006. PMID: 16677749 Review.
Cited by
- Withdrawal from long-term methamphetamine self-administration 'normalizes' neurometabolites in rhesus monkeys: a (1) H MR spectroscopy study.
Yang S, Belcher AM, Chefer S, Vaupel DB, Schindler CW, Stein EA, Yang Y. Yang S, et al. Addict Biol. 2015 Jan;20(1):69-79. doi: 10.1111/adb.12078. Epub 2013 Aug 4. Addict Biol. 2015. PMID: 23910722 Free PMC article. - Impaired glutamate homeostasis in the nucleus accumbens in human cocaine addiction.
Engeli EJE, Zoelch N, Hock A, Nordt C, Hulka LM, Kirschner M, Scheidegger M, Esposito F, Baumgartner MR, Henning A, Seifritz E, Quednow BB, Herdener M. Engeli EJE, et al. Mol Psychiatry. 2021 Sep;26(9):5277-5285. doi: 10.1038/s41380-020-0828-z. Epub 2020 Jun 29. Mol Psychiatry. 2021. PMID: 32601455 - N-acetylcysteine reduces cocaine-seeking behavior and anterior cingulate glutamate/glutamine levels among cocaine-dependent individuals.
Woodcock EA, Lundahl LH, Khatib D, Stanley JA, Greenwald MK. Woodcock EA, et al. Addict Biol. 2021 Mar;26(2):e12900. doi: 10.1111/adb.12900. Epub 2020 Mar 24. Addict Biol. 2021. PMID: 32212237 Free PMC article. Clinical Trial. - Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain.
Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Chang L, et al. J Neuroimmune Pharmacol. 2013 Jun;8(3):576-93. doi: 10.1007/s11481-013-9460-x. Epub 2013 May 12. J Neuroimmune Pharmacol. 2013. PMID: 23666436 Free PMC article. Review. - Phase-adjusted echo time (PATE)-averaging 1 H MRS: application for improved glutamine quantification at 2.89 T.
Prescot AP, Richards T, Dager SR, Choi C, Renshaw PF. Prescot AP, et al. NMR Biomed. 2012 Nov;25(11):1245-52. doi: 10.1002/nbm.2795. Epub 2012 Mar 12. NMR Biomed. 2012. PMID: 22407923 Free PMC article.
References
- Adalsteinsson E, Hurd RE, Mayer D, Sailasuta N, Sullivan EV, Pfefferbaum A. In vivo 2D J-resolved magnetic resonance spectroscopy of rat brain with a 3-T clinical human scanner. Neuroimage. 2004;22:381–386. - PubMed
- Adalsteinsson E, Sullivan EV, Mayer D, Pfefferbaum A. In vivo quantification of ethanol kinetics in rat brain. Neuropsychopharmacology. 2006;31:2683–2691. - PubMed
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nature Neuroscience. 2003;6:743–749. - PubMed
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996.
- Chang L, Ernst T, Strickland T, Mehringer CM. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry. 1999;156:716–722. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical