Conformational changes and signaling in cell and matrix physics - PubMed (original) (raw)
Review
Conformational changes and signaling in cell and matrix physics
André E X Brown et al. Curr Biol. 2009.
Abstract
Physical factors drive evolution and play important roles in motility and attachment as well as in differentiation. As animal cells adhere to survive, they generate force and 'feel' various mechanical features of their surroundings, with mechanosensory mechanisms based in part on force-induced conformational changes. Single-molecule methods for in vitro nano-manipulation, together with new in situ proteomic approaches that exploit mass spectrometry, are helping to identify and characterize the molecules and mechanics of structural transitions within cells and matrices. Given the diversity of cell and molecular responses, networks of biomolecules with conformations and interactions sculpted by force seem more likely than singular mechanosensors. Elaboration of the proteins that unfold and change structure in the extracellular matrix and in cells is needed - particularly with regard to the force-driven kinetics - in order to understand the systems biology of signaling in development, differentiation, and disease.
Figures
Figure 1
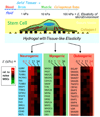
Cells can ‘feel’ the physical properties of their microenvironment. In one recent example with mesenchymal stem cells, matrix elasticity is seen to direct lineage specification [5]. Inhibition of the cell’s contractile tension blocks mechanosensation.
Figure 2
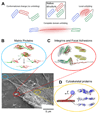
(A) When force is applied to proteins their native structure can be perturbed. This can involve a conformational change in which only quaternary structure is perturbed or unfolding in which secondary or tertiary structure are disrupted locally or over an entire domain. Lower panel: Adherent cells attach to the extracellular matrix (ECM), spread, and apply contractile forces using acto-myosin stress fibers. The image in the lower left shows the detergent extracted cytoskeleton of a mesenchymal stem cell adhering to its substrate. This can in principle activate several force sensitive processes that may be mediated by protein unfolding. (B) ECM proteins such as fibronectin are extended, exposing cryptic binding sites that promote fiber assembly as well as cell adhesion (inset adapted from [23], permission pending). (C) Focal adhesions sense applied force and respond by recruiting additional proteins to shore up the cell-substrate interaction. A possible contributing mechanism is the force-induced exposure of vinculin binding sites in the talin rod domain, shown here to unfold under force in molecular dynamics simulations (adapted from [55], permission pending). (D) The cytoskeleton is actively contractile and these forces impact not only the ECM and focal adhesions, but also the cytoskeleton itself. The inset shows simulations of the forced unfolding of an actin binding protein called filamin (adapted from [76], permission pending). The schematic (adapted with permission from original by Hyungsuk Lee, Jorge M. Ferrer, and Matthew J. Lang) to the left shows how filamin unfolding and actin unbinding can be simultaneously studied in single molecule experiments [78].
Figure 3
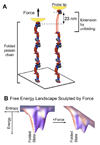
Nano-tools for protein folding and funnels. (A) Proteins can be extended and unfolded by force using a probe (shown in yellow) either in AFM or optical tweezers (adapted from [33], permission pending). (B) The energy of an extended protein conformation is typically higher than the folded native state and this is often conceptualized as an energy landscape in the form of a folding funnel where chain entropy dominates with the many unfolded states and energetic interactions pull the protein into one or a few well-defined structures. With force, the energy of more extended conformations is decreased, thereby accelerating unfolding.
Figure 4
(A) Cysteines are either buried within a protein fold or interaction site, or else they are exposed on the protein surface. The latter surface sites are rapidly labeled with one color before perturbation while the buried sites are then exposed and colored differently. This provides a ratiometric signal of unfolding and improves signal-to-noise ratio for exposed sites. (B) Addition of a membrane-permeable dye to adherent cells (in this case mesenchymal stem cells) labels cysteines depending on cell state, and relaxation of myosin (here with blebbistatin) leads to significant differences in protein labeling. Proteins that remain folded or assembled upon relaxation show a site-specific decrease in cysteine accessibility that is pinpointed using mass spectrometry (adapted from [9], permission pending).
Similar articles
- Molecular architecture and function of matrix adhesions.
Geiger B, Yamada KM. Geiger B, et al. Cold Spring Harb Perspect Biol. 2011 May 1;3(5):a005033. doi: 10.1101/cshperspect.a005033. Cold Spring Harb Perspect Biol. 2011. PMID: 21441590 Free PMC article. Review. - Transmembrane crosstalk between the extracellular matrix--cytoskeleton crosstalk.
Geiger B, Bershadsky A, Pankov R, Yamada KM. Geiger B, et al. Nat Rev Mol Cell Biol. 2001 Nov;2(11):793-805. doi: 10.1038/35099066. Nat Rev Mol Cell Biol. 2001. PMID: 11715046 Review. - Extracellular matrix in development: insights from mechanisms conserved between invertebrates and vertebrates.
Brown NH. Brown NH. Cold Spring Harb Perspect Biol. 2011 Dec 1;3(12):a005082. doi: 10.1101/cshperspect.a005082. Cold Spring Harb Perspect Biol. 2011. PMID: 21917993 Free PMC article. Review. - Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics.
Kumar S, Maxwell IZ, Heisterkamp A, Polte TR, Lele TP, Salanga M, Mazur E, Ingber DE. Kumar S, et al. Biophys J. 2006 May 15;90(10):3762-73. doi: 10.1529/biophysj.105.071506. Epub 2006 Feb 24. Biophys J. 2006. PMID: 16500961 Free PMC article. - Integrins, tensegrity, and mechanotransduction.
Ingber DE. Ingber DE. Gravit Space Biol Bull. 1997 Jun;10(2):49-55. Gravit Space Biol Bull. 1997. PMID: 11540119 Review.
Cited by
- Real-time observation of flow-induced cytoskeletal stress in living cells.
Rahimzadeh J, Meng F, Sachs F, Wang J, Verma D, Hua SZ. Rahimzadeh J, et al. Am J Physiol Cell Physiol. 2011 Sep;301(3):C646-52. doi: 10.1152/ajpcell.00099.2011. Epub 2011 Jun 8. Am J Physiol Cell Physiol. 2011. PMID: 21653900 Free PMC article. - Role of fluid dynamics and inflammation in intracranial aneurysm formation.
Turjman AS, Turjman F, Edelman ER. Turjman AS, et al. Circulation. 2014 Jan 21;129(3):373-82. doi: 10.1161/CIRCULATIONAHA.113.001444. Circulation. 2014. PMID: 24446407 Free PMC article. Review. - High refractive index silicone gels for simultaneous total internal reflection fluorescence and traction force microscopy of adherent cells.
Gutierrez E, Tkachenko E, Besser A, Sundd P, Ley K, Danuser G, Ginsberg MH, Groisman A. Gutierrez E, et al. PLoS One. 2011;6(9):e23807. doi: 10.1371/journal.pone.0023807. Epub 2011 Sep 22. PLoS One. 2011. PMID: 21961031 Free PMC article. - Mechanical perturbation of filamin A immunoglobulin repeats 20-21 reveals potential non-equilibrium mechanochemical partner binding function.
Chen H, Chandrasekar S, Sheetz MP, Stossel TP, Nakamura F, Yan J. Chen H, et al. Sci Rep. 2013;3:1642. doi: 10.1038/srep01642. Sci Rep. 2013. PMID: 23571456 Free PMC article. - Interplay between cytoskeletal stresses and cell adaptation under chronic flow.
Verma D, Ye N, Meng F, Sachs F, Rahimzadeh J, Hua SZ. Verma D, et al. PLoS One. 2012;7(9):e44167. doi: 10.1371/journal.pone.0044167. Epub 2012 Sep 19. PLoS One. 2012. PMID: 23028495 Free PMC article.
References
- Pelling AE, Horton MA. An historical perspective on cell mechanics. Pflügers Arch. 2008;456:3–12. - PubMed
- Wirtz D. Particle Tracking Microrheology of Living Cells: Principles and Applications. Ann. Rev. Biophys. 2009;38 - PubMed
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Bio. 2001;153:1175–1186. - PMC - PubMed
- Discher D, Janmey P, Wang Y. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. - PubMed
- Engler AJ, Sen S, Sweeney HL, Discher D. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 DK032094-21/DK/NIDDK NIH HHS/United States
- P01 DK032094-22/DK/NIDDK NIH HHS/United States
- R01 HL062352-09A1/HL/NHLBI NIH HHS/United States
- R01 HL062352/HL/NHLBI NIH HHS/United States
- P01 DK032094-24A15589/DK/NIDDK NIH HHS/United States
- P01 DK032094-24A1/DK/NIDDK NIH HHS/United States
- R21 AR056128-01A1/AR/NIAMS NIH HHS/United States
- P01 DK032094-23/DK/NIDDK NIH HHS/United States
- R21 AR056128/AR/NIAMS NIH HHS/United States
- S10 RR022575-01A1/RR/NCRR NIH HHS/United States
- R21 AR056128-02/AR/NIAMS NIH HHS/United States
- P01 DK032094-25/DK/NIDDK NIH HHS/United States
- R01 HL062352-10/HL/NHLBI NIH HHS/United States
- S10 RR022575/RR/NCRR NIH HHS/United States
- P01 DK032094-20/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials