Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy - PubMed (original) (raw)
Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy
Wei Zhang et al. J Neurosci. 2009.
Abstract
In temporal lobe epilepsy, seizures initiate in or near the hippocampus, which frequently displays loss of neurons, including inhibitory interneurons. It is unclear whether surviving interneurons function normally, are impaired, or develop compensatory mechanisms. We evaluated GABAergic interneurons in the hilus of the dentate gyrus of epileptic pilocarpine-treated GIN mice, specifically a subpopulation of somatostatin interneurons that expresses enhanced green fluorescence protein (GFP). GFP-immunocytochemistry and stereological analyses revealed substantial loss of GFP-positive hilar neurons (GPHNs) but increased GFP-positive axon length per dentate gyrus in epileptic mice. Individual biocytin-labeled GPHNs in hippocampal slices from epileptic mice also had larger somata, more axon in the molecular layer, and longer dendrites than controls. Dual whole-cell patch recording was used to test for monosynaptic connections from hilar GPHNs to granule cells. Unitary IPSCs (uIPSCs) recorded in control and epileptic mice had similar average rise times, amplitudes, charge transfers, and decay times. However, the probability of finding monosynaptically connected pairs and evoking uIPSCs was 2.6 times higher in epileptic mice compared to controls. Together, these findings suggest that surviving hilar somatostatin interneurons enlarge, extend dendrites, sprout axon collaterals in the molecular layer, and form new synapses with granule cells. These epilepsy-related changes in cellular morphology and connectivity may be mechanisms for surviving hilar interneurons to inhibit more granule cells and compensate for the loss of vulnerable interneurons.
Figures
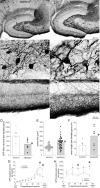
Figure 1.
GFP-immunoreactivity in the dentate gyrus of a control (left) and epileptic pilocarpine-treated GIN mouse (right). A, Within the dentate gyrus, more GFP-positive neurons are evident in the hilus (h) of the control compared to the epileptic mouse. g, Granule cell layer; m, molecular layer; CA3, CA3 pyramidal cell layer. B, High-magnification views of circled regions in A reveal larger GFP-positive somata in the epileptic mouse. C, High-magnification views of regions indicated by asterisks in A reveal more intensely labeled GFP-positive axons extending through the granule cell layer and ramifying within the molecular layer, especially the outer molecular layer, in the epileptic mouse. D, The average number of GFP-positive hilar neurons per dentate gyrus (bars) was reduced in epileptic mice (*p = 0.01, t test, n = 8 controls, 8 epileptic mice). E, The average soma area (bars) was increased in epileptic mice (*p = 0.001, t test). Symbols indicate values of each measured soma. Many somata in epileptic mice exceeded the maximum of control mice. F, The average cumulative length of GFP-positive axon in the granule cell layer plus molecular layer per dentate gyrus (bars) was increased in epileptic mice (*p = 0.02, t test). G, GFP-positive hilar neurons were more numerous in sections from the temporal hippocampus. Epileptic mice had fewer neurons/section at all septotemporal levels, but especially in the temporal hippocampus. Values indicate mean ± SEM, *p < 0.001, repeated-measures ANOVA, Holm–Sidak method. H, Despite neuron loss, GFP-positive axon length per section was greater at all septotemporal levels of the hippocampus in epileptic mice. *p < 0.05, repeated-measures ANOVA, Holm–Sidak method.
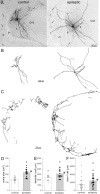
Figure 2.
Biocytin-labeled GFP-positive hilar somatostatin interneurons in a control (left) and epileptic pilocarpine-treated GIN mouse (right). A, Biocytin-labeled GFP-positive neurons in the hilus (h) of 300 μm thick slices. Arrows indicate axon in the molecular layer (m). g, Granule cell layer; CA3, CA3 pyramidal cell layer. B, Soma and dendrite reconstructions of cells shown in A. C, Axon reconstructions in the molecular layer of cells shown in A. Only axon in the molecular layer, not hilus or granule cell layer, was reconstructed. D, Average soma area (bars) was increased in epileptic mice (*p < 0.0001, t test, n = 16 controls, 19 epileptic mice). E, Average dendritic length per cell (bars) was increased in epileptic mice (*p = 0.02, t test). F, Average axon length in the dentate gyrus molecular layer per cell (bars) was increased in epileptic mice (*p < 0.05, t test, n = 25 control, 26 epileptic mice).
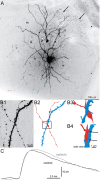
Figure 3.
uIPSCs recorded in granule cells and generated by green fluorescent protein-positive hilar somatostatin interneurons in slices from control and epileptic pilocarpine-treated GIN mice. A, A biocytin-labeled interneuron in the hilus (h) and granule cell (arrowhead) in a slice from an epileptic mouse. The interneuron's axon (arrows) is concentrated in the molecular layer (m). g, Granule cell layer. B, An apposition of the interneuron's axon and the granule cell's dendrite. B1, High-magnification view of the region indicated by the white circle in A showing the spiny granule cell dendrite and interneuron axon with varicosities. B2, Reconstruction of the granule cell dendrite (blue) and interneuron axon (red). B3, High-magnification view of the area in the rectangle in B2. B4, Side view with axon-dendrite appositions indicated by arrows. C, Average uIPSCs from a control (black) and epileptic mouse (gray).
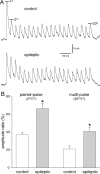
Figure 4.
Paired- and multipulse depression was reduced at interneuron-to-granule cell synapses in epileptic mice. A, Trains of uIPSCs recorded in granule cells and generated by green fluorescent protein-positive hilar somatostatin interneurons in slices from control and epileptic pilocarpine-treated GIN mice. Action potentials (20) were evoked in interneurons at 50 Hz every 10 s. At least 20 traces were recorded and averaged. Average amplitudes of the first, second, and 20th uIPSCs were measured. B, Paired- and multipulse amplitude ratios were larger in epileptic mice (*p < 0.009, t test, n = 8 control, 15 epileptic mice).
Similar articles
- Rapamycin suppresses axon sprouting by somatostatin interneurons in a mouse model of temporal lobe epilepsy.
Buckmaster PS, Wen X. Buckmaster PS, et al. Epilepsia. 2011 Nov;52(11):2057-64. doi: 10.1111/j.1528-1167.2011.03253.x. Epub 2011 Aug 29. Epilepsia. 2011. PMID: 21883182 Free PMC article. - Hilar somatostatin interneuron loss reduces dentate gyrus inhibition in a mouse model of temporal lobe epilepsy.
Hofmann G, Balgooyen L, Mattis J, Deisseroth K, Buckmaster PS. Hofmann G, et al. Epilepsia. 2016 Jun;57(6):977-83. doi: 10.1111/epi.13376. Epub 2016 Mar 31. Epilepsia. 2016. PMID: 27030321 Free PMC article. - Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy.
Kobayashi M, Buckmaster PS. Kobayashi M, et al. J Neurosci. 2003 Mar 15;23(6):2440-52. doi: 10.1523/JNEUROSCI.23-06-02440.2003. J Neurosci. 2003. PMID: 12657704 Free PMC article. - The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy.
Sloviter RS. Sloviter RS. Ann Neurol. 1994 Jun;35(6):640-54. doi: 10.1002/ana.410350604. Ann Neurol. 1994. PMID: 8210220 Review. - Neuroplasticity in the damaged dentate gyrus of the epileptic brain.
Ribak CE, Dashtipour K. Ribak CE, et al. Prog Brain Res. 2002;136:319-28. doi: 10.1016/s0079-6123(02)36027-8. Prog Brain Res. 2002. PMID: 12143392 Review.
Cited by
- Neonatal estradiol stimulation prevents epilepsy in Arx model of X-linked infantile spasms syndrome.
Olivetti PR, Maheshwari A, Noebels JL. Olivetti PR, et al. Sci Transl Med. 2014 Jan 22;6(220):220ra12. doi: 10.1126/scitranslmed.3007231. Sci Transl Med. 2014. PMID: 24452264 Free PMC article. - Peripheral Nerve Injury Induces Changes in the Activity of Inhibitory Interneurons as Visualized in Transgenic GAD1-GCaMP6s Rats.
Krishnan V, Wade-Kleyn LC, Israeli RR, Pelled G. Krishnan V, et al. Biosensors (Basel). 2022 Jun 1;12(6):383. doi: 10.3390/bios12060383. Biosensors (Basel). 2022. PMID: 35735531 Free PMC article. - Decrease in CA3 inhibitory network activity during Theiler's virus encephalitis.
Smeal RM, Fujinami R, White HS, Wilcox KS. Smeal RM, et al. Neurosci Lett. 2015 Nov 16;609:210-5. doi: 10.1016/j.neulet.2015.10.032. Epub 2015 Oct 23. Neurosci Lett. 2015. PMID: 26477780 Free PMC article. - Remodeling of dendrites and spines in the C1q knockout model of genetic epilepsy.
Ma Y, Ramachandran A, Ford N, Parada I, Prince DA. Ma Y, et al. Epilepsia. 2013 Jul;54(7):1232-9. doi: 10.1111/epi.12195. Epub 2013 Apr 26. Epilepsia. 2013. PMID: 23621154 Free PMC article. - Somatostatin-expressing neurons in cortical networks.
Urban-Ciecko J, Barth AL. Urban-Ciecko J, et al. Nat Rev Neurosci. 2016 Jul;17(7):401-9. doi: 10.1038/nrn.2016.53. Epub 2016 May 26. Nat Rev Neurosci. 2016. PMID: 27225074 Free PMC article. Review.
References
- Abusaad I, MacKay D, Zhao J, Stanford P, Collier DA, Everall IP. Stereological estimates of the total number of neurons in the murine hippocampus using the optical dissector. J Comp Neurol. 1999;408:560–566. - PubMed
- André V, Marescaux C, Nehlig A, Fritschy JM. Alterations of hippocampal GABA system contribute to development of spontaneous recurrent seizures in the rat lithiumpilocarpine model of temporal lobe epilepsy. Hippocampus. 2001;11:452–468. - PubMed
- Arellano JI, Muñoz A, Ballesteros-Yáñez I, Sola RG, DeFelipe J. Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain. 2004;127:45–64. - PubMed
- Austin JE, Buckmaster PS. Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. J Comp Neurol. 2004;476:205–218. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T35 RR017188/RR/NCRR NIH HHS/United States
- T35 RR017188-08/RR/NCRR NIH HHS/United States
- R01 NS039110/NS/NINDS NIH HHS/United States
- T35 OD010989/OD/NIH HHS/United States
- R01 NS039579-11/NS/NINDS NIH HHS/United States
- R01 NS039110-09/NS/NINDS NIH HHS/United States
- R01 NS039579/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources