Evolutionary concepts in biobanking - the BC BioLibrary - PubMed (original) (raw)
doi: 10.1186/1479-5876-7-95.
Janet E Wilson-McManus, Rebecca O Barnes, Sara C Giesz, Adrian Png, Richard G Hegele, Jacquelyn N Brinkman, Ian R Mackenzie, David G Huntsman, Anne Junker, Blake Gilks, Erik Skarsgard, Michael Burgess, Samuel Aparicio, Bruce M McManus
Affiliations
- PMID: 19909513
- PMCID: PMC2785772
- DOI: 10.1186/1479-5876-7-95
Evolutionary concepts in biobanking - the BC BioLibrary
Peter H Watson et al. J Transl Med. 2009.
Abstract
Background: Medical research to improve health care faces a major problem in the relatively limited availability of adequately annotated and collected biospecimens. This limitation is creating a growing gap between the pace of scientific advances and successful exploitation of this knowledge. Biobanks are an important conduit for transfer of biospecimens (tissues, blood, body fluids) and related health data to research. They have evolved outside of the historical source of tissue biospecimens, clinical pathology archives. Research biobanks have developed advanced standards, protocols, databases, and mechanisms to interface with researchers seeking biospecimens. However, biobanks are often limited in their capacity and ability to ensure quality in the face of increasing demand. Our strategy to enhance both capacity and quality in research biobanking is to create a new framework that repatriates the activity of biospecimen accrual for biobanks to clinical pathology.
Methods: The British Columbia (BC) BioLibrary is a framework to maximize the accrual of high-quality, annotated biospecimens into biobanks. The BC BioLibrary design primarily encompasses: 1) specialized biospecimen collection units embedded within clinical pathology and linked to a biospecimen distribution system that serves biobanks; 2) a systematic process to connect potential donors with biobanks, and to connect biobanks with consented biospecimens; and 3) interdisciplinary governance and oversight informed by public opinion.
Results: The BC BioLibrary has been embraced by biobanking leaders and translational researchers throughout BC, across multiple health authorities, institutions, and disciplines. An initial pilot network of three Biospecimen Collection Units has been successfully established. In addition, two public deliberation events have been held to obtain input from the public on the BioLibrary and on issues including consent, collection of biospecimens and governance.
Conclusion: The BC BioLibrary framework addresses common issues for clinical pathology, biobanking, and translational research across multiple institutions and clinical and research domains. We anticipate that our framework will lead to enhanced biospecimen accrual capacity and quality, reduced competition between biobanks, and a transparent process for donors that enhances public trust in biobanking.
Figures
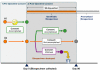
Figure 1
The BC BioLibrary and its components. The BC BioLibrary is a framework that lies upstream from biobanks in the cycle that begins and ends with people and leads to their better health. Specifically addressing the aspects of biobanking that involve collection and processing of biospecimens, the components include: 1) the Biospecimen Collection Units which are embedded in the hospital pathology departments and facilitates research orientated biospecimen processing by trained personnel using SOPs; 2) data management infrastructures which enable integration of consent information provided to biobanks with biospecimens from patient donors; and 3) public engagement processes to allow informed deliberation and input from the public into the governance of biobanking.
Figure 2
The possible status of biospecimens collected by the BC BioLibrary BCU, as determined by the consent linked to the biospecimen in relation to the time of surgery. The consent status of biospecimens collected and held by the BCU is influenced by two possible mechanisms for consent: #1) Pre-Operative Consent: If consent is secured pre-operatively by a biobank then the biospecimen (green circle) is collected by the BCU and distributed to the biobank as a coded but identifiable biospecimen that can be linked to the patient donor clinical data by the biobank. #2) Post-Operative Consent: If consent is to be sought post-operatively then the biospecimen is collected by the BCU and held as an identifiable biospecimen (orange circle) for a period of up to 90 days (orange lines). During this time the consent status of the biospecimen may change and allow distribution to a biobank as follows: Accomplished - biospecimen (green circle) is distributed as per the procedure following a Pre-Operative Consent process. Not accomplished - the biospecimen (grey circle) and all related collection data is anonymized and distributed to a biobank (if approved to receive such biospecimens) or destroyed Withheld - biospecimen (purple circle) and all related collection data is destroyed.
Similar articles
- Involving citizens in the ethics of biobank research: informing institutional policy through structured public deliberation.
O'Doherty KC, Hawkins AK, Burgess MM. O'Doherty KC, et al. Soc Sci Med. 2012 Nov;75(9):1604-11. doi: 10.1016/j.socscimed.2012.06.026. Epub 2012 Jul 28. Soc Sci Med. 2012. PMID: 22867865 - The Future of Biobanking: A Conceptual Look at How Biobanks Can Respond to the Growing Human Biospecimen Needs of Researchers.
Somiari SB, Somiari RI. Somiari SB, et al. Adv Exp Med Biol. 2015;864:11-27. doi: 10.1007/978-3-319-20579-3_2. Adv Exp Med Biol. 2015. PMID: 26420610 Review. - "It's all about trust": reflections of researchers on the complexity and controversy surrounding biobanking in South Africa.
Moodley K, Singh S. Moodley K, et al. BMC Med Ethics. 2016 Oct 10;17(1):57. doi: 10.1186/s12910-016-0140-2. BMC Med Ethics. 2016. PMID: 27724893 Free PMC article. - Informed consent in biobank research: a deliberative approach to the debate.
Secko DM, Preto N, Niemeyer S, Burgess MM. Secko DM, et al. Soc Sci Med. 2009 Feb;68(4):781-9. doi: 10.1016/j.socscimed.2008.11.020. Epub 2008 Dec 16. Soc Sci Med. 2009. PMID: 19095337 - Biobanking Comes of Age: The Transition to Biospecimen Science.
Vaught J. Vaught J. Annu Rev Pharmacol Toxicol. 2016;56:211-28. doi: 10.1146/annurev-pharmtox-010715-103246. Epub 2015 Oct 22. Annu Rev Pharmacol Toxicol. 2016. PMID: 26514206 Review.
Cited by
- Effects of ex vivo ischemia time and delayed processing on quality of specimens in tissue biobank.
Guo D, Wang A, Xie T, Zhang S, Cao D, Sun J. Guo D, et al. Mol Med Rep. 2020 Nov;22(5):4278-4288. doi: 10.3892/mmr.2020.11503. Epub 2020 Sep 10. Mol Med Rep. 2020. PMID: 33000275 Free PMC article. - Linkage of data from diverse data sources (LDS): a data combination model provides clinical data of corresponding specimens in biobanking information system.
Eminaga O, Özgür E, Semjonow A, Herden J, Akbarov I, Tok A, Engelmann U, Wille S. Eminaga O, et al. J Med Syst. 2013 Oct;37(5):9975. doi: 10.1007/s10916-013-9975-y. Epub 2013 Sep 11. J Med Syst. 2013. PMID: 24022214 - Impact of non-welfare interests on willingness to donate to biobanks: an experimental survey.
Gornick MC, Ryan KA, Kim SY. Gornick MC, et al. J Empir Res Hum Res Ethics. 2014 Oct;9(4):22-33. doi: 10.1177/1556264614544277. Epub 2014 Aug 11. J Empir Res Hum Res Ethics. 2014. PMID: 25747294 Free PMC article. Clinical Trial. - Co-occurrence of anaerobic bacteria in colorectal carcinomas.
Warren RL, Freeman DJ, Pleasance S, Watson P, Moore RA, Cochrane K, Allen-Vercoe E, Holt RA. Warren RL, et al. Microbiome. 2013 May 15;1(1):16. doi: 10.1186/2049-2618-1-16. Microbiome. 2013. PMID: 24450771 Free PMC article. - If you build it, they will come: unintended future uses of organised health data collections.
O'Doherty KC, Christofides E, Yen J, Bentzen HB, Burke W, Hallowell N, Koenig BA, Willison DJ. O'Doherty KC, et al. BMC Med Ethics. 2016 Sep 6;17(1):54. doi: 10.1186/s12910-016-0137-x. BMC Med Ethics. 2016. PMID: 27600117 Free PMC article. Review.
References
- Towbin JA, Bowles NE. The failing heart. Nature. 2002;415:227–233. - PubMed
- Strausberg RL, Simpson AJ, Old LJ, Riggins GJ. Oncogenomics and the development of new cancer therapies. Nature. 2004;429:469–474. - PubMed
- Topol EJ, Murray SS, Frazer KA. The genomics gold rush. Jama. 2007;298:218–221. - PubMed
- Korn D. In: Research Involving Human Biological Material:Ethical Issues and Policy Guidance. Commission NBA, editor. II. 2000. Contribution of the Human Tissue Archive to the Advancement of Medical Knowledge and the Public Health; pp. 1–30.
- Morente MM, Fernandez PL, de Alava E. Biobanking: old activity or young discipline? Semin Diagn Pathol. 2008;25:317–322. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous