Hsp90 Co-localizes with Rab-GDI-1 and regulates agonist-induced amylase release in AR42J cells - PubMed (original) (raw)
Hsp90 Co-localizes with Rab-GDI-1 and regulates agonist-induced amylase release in AR42J cells
Robert Raffaniello et al. Cell Physiol Biochem. 2009.
Abstract
Rab proteins are small GTPases required for vesicle trafficking through the secretory and endocytic pathways. Rab GDP-dissociation inhibitor (rab-GDI) regulates Rab protein function and localization by maintaining Rab proteins in the GDP-bound conformation. Two isoforms of rab-GDI are present in most mammalian cells: GDI-1 and GDI-2. It has recently been demonstrated that a Heat shock protein 90 (Hsp90) chaperone complex regulates the interactions between Rab proteins and Rab-GDI-1. The AR42J cell line is derived from rat pancreatic exocrine tumor cells and develops an acinar-like phenotype when treated with dexamethasone (Dex). The aim of the present study was to examine the expression of rab-GDI isoforms and Hsp90 in AR42J cells in the presence or absence of Dex. Rab-GDI:Hsp90 interactions were also examined. Both rab-GDI isoforms were detected in AR42J cells by immunoblotting. In Dex-treated cells, quantitative immunoblotting revealed that rab-GDI-1 expression increased by 28%, although this change was not statistically significant. Rab-GDI-2 levels were unaltered by Dex treatment. Approximately 21% rab-GDI-1 was membrane associated, whereas rab-GDI-2 was exclusively cytosolic. Dex treatment did not affect the subcellular distribution of rab-GDI isoforms. Hsp90 was present in the cytosolic and membrane fractions of AR42J cells and co-immunoprecipitated with cytosolic rab-GDI-1. Moreover, density gradient centrifugation of AR42J cell membranes revealed that Hsp90 and rab-GDI-1 co-localize on low- and high-density membrane fractions, including amylase-containing secretory granules. The Hsp90 inhibitor, geldanamycin, inhibited CCK-8-induced amylase release from these cells in a dose-dependent manner. Our results indicate that as AR42J cells differentiate into acinar-like cells, rab-GDI isoform expression and localization is not significantly altered. Moreover, our findings suggest that Hsp90 regulates agonist-induced secretion in exocrine cells by interacting with rab-GDI-1.
2009 S. Karger AG, Basel.
Figures
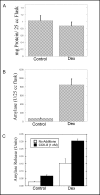
Fig. 1
Effect of Dex treatment on AR42J cells. AR42J cells were cultured in T-25 flasks in the absence (Control) or presence (Dex) of 50 nM Dex for 2-3 days. The cells were washed and harvested by scraping into PBS. The cells were then pelleted and resuspended in approximately 0.5 ml of lysing solution. Total protein (Fig. 1A) and amylase (Fig. 1B) levels in the flasks were determined as described in Methods. C. AR42J cells were seeded onto 6-well plates and cultured in the absence (Control) or presence (Dex) of 50 nM Dex for 2-3 days. On the day the cells were assayed, the media was removed and the cells washed with amylase assay buffer (AAB). The cells were then incubated with 1 nM CCK-8 or no additions for 40 min at 37oC. Amylase release from control and Dex-treated cells during the 40 min incubation was measured as described in methods. Values for amylase release are expressed as Amylase Units. Results shown are means ± SEM from at least three experiments.
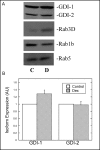
Fig. 2
Effect of Dex on the expression of rab-GDI isoforms and Rab proteins in AR42J cells. A. AR42J cells were grown in the absence (C) or presence (D) of Dex (50 nM) for 3 days. Equal amounts of protein from AR42J cell lysates were immunoblotted for Rab-GDI, Rab1B, Rab3D and Rab5 as described in Methods. B. Relative levels of rab-GDI isoforms in control and Dex-treated cells were determined by quantitative immunoblotting. The data are presented as arbitrary units. Results shown are means ± SEM from at least three experiments.
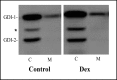
Fig. 3
Effect of Dex on the subcellular localization of rab-GDI isoforms in AR42J cells. AR42J cells were cultured in the absence (Control) or presence of Dex (50 nM) for 3 days. The cells were harvested and cytosol (C) and membrane (M) fractions were prepared and immunoblotted for rab-GDI isoforms using an antibody that recognizes both GDI-1 and GDI-2 as described in Methods. The asterisk indicates a non-specific band that is often observed between the two rab-GDI isoforms and most likely represents amylase.
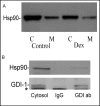
Fig. 4
Hsp90 is expressed in AR42J cells and associates with cytosolic rab-GDI-1. A. Hsp90 expression and localization in AR42J cells in the absence (Control) or presence (Dex) of Dex was examined. Cytosol (C) and membrane (M) fractions were prepared from control and Dex-treated cells and immunoblotted for Hsp90 as described in Methods. B. Immunoprecipitation of Rab-GDI-1 from AR42J cell cytosol. Cytosol was prepared from four T-75 flasks of Dex-treated AR42J cells. The cytosol was incubated overnight with a 30 μg GDI-1-specific antibody (GDI ab-right lane) or rabbit IgG (IgG-middle lane), followed by a three hr incubation with protein G-agarose beads. The beads were washed and boiled into SDS sample buffer as described in Methods. The cytosol (left lane) and immunoprecipitates were immunoblotted for Hsp90 and rab-GDI. The asterisk in the GDI-1 blot is a non-specific band and most likely represents amylase.
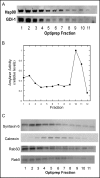
Fig. 5
Rab-GDI-1 and Hsp90 co-localize on AR42J cell membrane fractions. A. AR42J cells were treated with Dex for 2 days and harvested into lysing buffer. A post-nuclear supernatant (PNS) was prepared and applied to a 10-30% continuous iodixanol gradient as described in Methods. Fractions (150 μl) were collected from the top of the gradient and immunoblotted for rab-GDI-1 and Hsp90. B. Amylase levels in the fractions were determined and the data were normalized to 1, which represents the maximum level of amylase activity. C. Immunoblotting of gradient fractions for Rab3D, Rab5, Calnexin and syntaxin 6.
Fig. 6
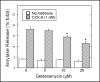
Geldanamycin inhibits CCK-8-induced amylase release. AR42J cells were plated in 6-well plates (3 × 105 cells/well) and treated with Dex (50 nM) for three days. The cells were washed with AAB and incubated in the presence or absence of the indicated concentration of geldanamycin for 30 min, washed once with AAB and incubated with no additions or CCK-8 (1 nM) for 40 min. Values for amylase release are expressed as percentage of the total cellular amylase present at the start of the 40 min incubation that was released into the medium. Results shown are means ± SEM from four experiments.
Similar articles
- Use of Hsp90 inhibitors to disrupt GDI-dependent Rab recycling.
Chen CY, Sakisaka T, Balch WE. Chen CY, et al. Methods Enzymol. 2005;403:339-47. doi: 10.1016/S0076-6879(05)03029-6. Methods Enzymol. 2005. PMID: 16473600 - The Hsp90 chaperone complex regulates GDI-dependent Rab recycling.
Chen CY, Balch WE. Chen CY, et al. Mol Biol Cell. 2006 Aug;17(8):3494-507. doi: 10.1091/mbc.e05-12-1096. Epub 2006 May 10. Mol Biol Cell. 2006. PMID: 16687576 Free PMC article. - Rab3D regulates amylase levels, not agonist-induced amylase release, in AR42J cells.
Limi S, Ojakian G, Raffaniello R. Limi S, et al. Cell Mol Biol Lett. 2012 Jun;17(2):258-73. doi: 10.2478/s11658-012-0008-5. Epub 2012 Feb 24. Cell Mol Biol Lett. 2012. PMID: 22367855 Free PMC article. - Molecular control of Rab activity by GEFs, GAPs and GDI.
Müller MP, Goody RS. Müller MP, et al. Small GTPases. 2018 Mar 4;9(1-2):5-21. doi: 10.1080/21541248.2016.1276999. Epub 2017 Feb 1. Small GTPases. 2018. PMID: 28055292 Free PMC article. Review. - Rab GTPase localization and Rab cascades in Golgi transport.
Pfeffer SR. Pfeffer SR. Biochem Soc Trans. 2012 Dec 1;40(6):1373-7. doi: 10.1042/BST20120168. Biochem Soc Trans. 2012. PMID: 23176483 Free PMC article. Review.
Cited by
- A Sensitized Screen for Genes Promoting Invadopodia Function In Vivo: CDC-42 and Rab GDI-1 Direct Distinct Aspects of Invadopodia Formation.
Lohmer LL, Clay MR, Naegeli KM, Chi Q, Ziel JW, Hagedorn EJ, Park JE, Jayadev R, Sherwood DR. Lohmer LL, et al. PLoS Genet. 2016 Jan 14;12(1):e1005786. doi: 10.1371/journal.pgen.1005786. eCollection 2016 Jan. PLoS Genet. 2016. PMID: 26765257 Free PMC article. - GDI2 is a novel diagnostic and prognostic biomarker in hepatocellular carcinoma.
Zhang W, Liu Z, Xia S, Yao L, Li L, Gan Z, Tang H, Guo Q, Yan X, Sun Z. Zhang W, et al. Aging (Albany NY). 2021 Dec 11;13(23):25304-25324. doi: 10.18632/aging.203748. Epub 2021 Dec 11. Aging (Albany NY). 2021. PMID: 34894398 Free PMC article. - Establishment of a novel, eco-friendly transgenic pig model using porcine pancreatic amylase promoter-driven fungal cellulase transgenes.
Lin YS, Yang CC, Hsu CC, Hsu JT, Wu SC, Lin CJ, Cheng WT. Lin YS, et al. Transgenic Res. 2015 Feb;24(1):61-71. doi: 10.1007/s11248-014-9817-9. Epub 2014 Jul 26. Transgenic Res. 2015. PMID: 25063310 - Silencing GDI2 inhibits proliferation, migration and invasion of colorectal cancer through activation of p53 signaling pathway.
Ou WT, Tan RJ, Zhai JW, Sun LJ, Xu FP, Huang XJ, Quan ZH, Zhou CJ. Ou WT, et al. Heliyon. 2024 Sep 13;10(18):e37770. doi: 10.1016/j.heliyon.2024.e37770. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39323841 Free PMC article. - Effect of ephrin-A1/EphA2 on invasion of trophoblastic cells.
Yang Y, Min J. Yang Y, et al. J Huazhong Univ Sci Technolog Med Sci. 2011 Dec;31(6):824-827. doi: 10.1007/s11596-011-0684-9. Epub 2011 Dec 16. J Huazhong Univ Sci Technolog Med Sci. 2011. PMID: 22173506
References
- Jordens I, Marsman M, Kuijl C, Neefjes J. Rab proteins, connecting transport and vesicle fusion. Traffic. 2005;6(12):1070–1077. - PubMed
- Burton J, De Camilli P. A novel mammalian guanine nucleotide exchange factor (GEF) specific for rab proteins. Adv Second Messenger Phosphoprotein Res. 1994;29:109–119. - PubMed
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. - PubMed
- Collins RN. “Getting it on“-GDI displacement and small GTPase membrane recruitment. Mol Cell. 2003;12(5):1064–1066. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous