A novel effect of rivastigmine on pre-synaptic proteins and neuronal viability in a neurodegeneration model of fetal rat primary cortical cultures and its implication in Alzheimer's disease - PubMed (original) (raw)
A novel effect of rivastigmine on pre-synaptic proteins and neuronal viability in a neurodegeneration model of fetal rat primary cortical cultures and its implication in Alzheimer's disease
Jason A Bailey et al. J Neurochem. 2010 Feb.
Abstract
Alzheimer's disease (AD) is characterized by deposition of amyloid-beta peptide plaque, disrupted amyloid-beta-precursor protein (APP) metabolism, hyperphosphorylation of Tau leading to neurofibrillary tangles and associated neurotoxicity. Moreover, there is synaptic loss in AD, which occurs early and may precede frank amyloidosis. The central cholinergic system is especially vulnerable to the toxic events associated with AD, and reduced acetylcholine levels in specific brain regions is thought to be central to memory deficits in AD. First-generation cholinesterase inhibitors have provided only symptomatic relief to patients with AD by prolonging the action of remaining acetylcholine with little or no change in the course of the disease. Some second-generation cholinesterase inhibitors are multifunctional drugs that may provide more than purely palliative results. To evaluate the effects of the dual acetylcholinesterase and butyrylcholinesterase inhibitor rivastigmine on key aspects of AD, embryonic day 16 rat primary cortical cultures were treated with rivastigmine under media conditions observed to induce time-dependent neurodegeneration. Samples were subjected to western blotting and immunocytochemistry techniques to determine what influence this drug may have on synaptic proteins and neuronal morphology. There was a strong increase in relative cell viability associated with rivastigmine treatment. Significant dose-dependent increases were observed in the levels of synaptic markers synaptosomal-associated protein of 25 kDa (SNAP-25) and synaptophysin, as well as the neuron-specific form of enolase. Together with an observed enhancement of neuronal morphology, our results suggest a rivastigmine-mediated novel neuroprotective and/or neurorestorative effects involving the synapse. Our observations may explain the potential for rivastigmine to alter the course of AD, and warrant further investigations into using butyrylcholinesterase inhibition as a therapeutic strategy for AD, especially with regard to restoration of synaptic function.
Figures
Figure 1
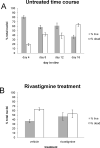
Nuclei were labeled with the fluorescent dyes DAPI and EthD-1 to produce total cell counts and dead cell counts, respectively. A. The untreated time course shows a time-dependent decrease in live cells, and increasing numbers of dead cells. B. 10μM rivastigmine treatment from day 12-16 produced higher live and lower dead cell counts, though this effect did not reach significance.
Figure 2
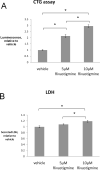
Cell viability was assessed using two different biochemical assays. After treatment, media samples were collected and cells were scraped from the plates and suspended in DPBS. A. Aliquots of the cell suspension were subjected to the Cell Titer-Glo assay. A clear, dose-dependent increase in cell viability (p<0.01) with rivastigmine treatment was observed. Rivastigmine produced 214% and 295% increases from vehicle at 5μM and 10μM, respectively. B. Cell culture media samples were subjected to the lactate dehydrogenase (LDH) assay to detect any toxic effects of treatment. Rivastigmine treatment produced a small 18% increase in the 10μM condition, however this is likely to be increased background LDH release due to increased cell survival in these treatments.
Figure 3
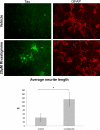
Neuronal cultures were treated with vehicle or 20μM rivastigmine for 24 hours starting at day 12 in vitro. These cultures were then fixed and labeled with anti-tau (green) and anti-GFAP (red) antibodies using standard immunocytochemical techniques. Few tau-positive cells and sparse neurites were observed in the vehicle treatment, while clusters of cells in the rivastigmine treatment showed strong tau labeling and a greater number of neurites. Quantification of neuronal morphology using the modified Ronn technique shows a significant, approximately 3-fold increase in neurite length (p=0.03).
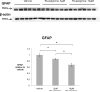
Figure 4
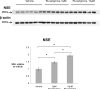
Probing of Western blots of treated lysates of cultures treated with 5μM or 10μM rivastigmine with an anti-NSE antibody shows a dose-dependent increase with rivastigmine treatment. 5μM and 10μM rivastigmine produced 1.5- and 2-fold increases in this neuronal marker, respectively, compared to vehicle (both p<0.01). This indicates a robust preservation of neurons in this mixed culture.
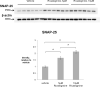
Figure 5
Probing Western blots of treated lysates with an antibody against the synaptic protein SNAP-25 shows that this protein increased 1.6- and 2.1-fold compared to vehicle in response to 5μM and 10μM rivastigmine, respectively (both p<0.01). These data suggest that rivastigmine may preserve synaptic terminals in neuronal cultures.
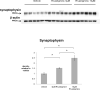
Figure 6
Probing Western blots of treated lysates with an antibody against the synaptic protein synaptophysin shows that this protein increased 1.5- and 2-fold compared to vehicle in reasponse to 5μM and 10μM rivastigmine, respectively (both p<0.01). Synaptophysin loss has been clearly associated with cognitive decline in AD, and these data suggest a synaptotrophic effect that could represent a disease-modifying property of rivastigmine.
Figure 7
Probing Western blots of treated lysates with an antibody against GFAP shows a dose-dependent decrease of 13% and 32% in response to 5μM and 10μM rivastigmine, respectively, compared to vehicle (both p<0.05). While these data appear to disagree with the immunocytochemistry data wherein glia appear to be unaffected by rivastigmine, we conclude that these decreases are the result of a greater representation of neuronal protein in the lysates of treated cultures.
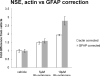
Figure 8
The Western blot data for NSE were recalculated to compare the levels of this protein as a proportion of GFAP to the actin ratio reported above (figure 4). As compared to vehicle, 5μM and 10μM rivastigmine produced 1.5- and 1.9-fold increases in NSE when these data are corrected with actin. When corrected with GFAP, however, rivastigmine produces 1.6- and 2.8-fold increases in NSE. This difference is likely due to the apparent decrease in glial protein represented in these lysates, which accentuates the dose-dependent increase in NSE.
Similar articles
- Rivastigmine lowers Aβ and increases sAPPα levels, which parallel elevated synaptic markers and metabolic activity in degenerating primary rat neurons.
Bailey JA, Ray B, Greig NH, Lahiri DK. Bailey JA, et al. PLoS One. 2011;6(7):e21954. doi: 10.1371/journal.pone.0021954. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799757 Free PMC article. - Oxidative insults to neurons and synapse are prevented by aged garlic extract and S-allyl-L-cysteine treatment in the neuronal culture and APP-Tg mouse model.
Ray B, Chauhan NB, Lahiri DK. Ray B, et al. J Neurochem. 2011 May;117(3):388-402. doi: 10.1111/j.1471-4159.2010.07145.x. Epub 2011 Mar 14. J Neurochem. 2011. PMID: 21166677 Free PMC article. - Selenium compounds prevent amyloid β-peptide neurotoxicity in rat primary hippocampal neurons.
Godoi GL, de Oliveira Porciúncula L, Schulz JF, Kaufmann FN, da Rocha JB, de Souza DO, Ghisleni G, de Almeida HL Jr. Godoi GL, et al. Neurochem Res. 2013 Nov;38(11):2359-63. doi: 10.1007/s11064-013-1147-4. Epub 2013 Sep 8. Neurochem Res. 2013. PMID: 24013888 - The cholinergic system in aging and neuronal degeneration.
Schliebs R, Arendt T. Schliebs R, et al. Behav Brain Res. 2011 Aug 10;221(2):555-63. doi: 10.1016/j.bbr.2010.11.058. Epub 2010 Dec 9. Behav Brain Res. 2011. PMID: 21145918 Review. - Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease.
Polinsky RJ. Polinsky RJ. Clin Ther. 1998 Jul-Aug;20(4):634-47. doi: 10.1016/s0149-2918(98)80127-6. Clin Ther. 1998. PMID: 9737824 Review.
Cited by
- Functional characterization of a competitive peptide antagonist of p65 in human macrophage-like cells suggests therapeutic potential for chronic inflammation.
Srinivasan M, Blackburn C, Lahiri DK. Srinivasan M, et al. Drug Des Devel Ther. 2014 Dec 3;8:2409-21. doi: 10.2147/DDDT.S59722. eCollection 2014. Drug Des Devel Ther. 2014. PMID: 25584020 Free PMC article. - Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats.
Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Ding ZM, et al. Addict Biol. 2013 Mar;18(2):297-306. doi: 10.1111/adb.12018. Epub 2012 Dec 14. Addict Biol. 2013. PMID: 23240885 Free PMC article. - Therapeutic roles of plants for 15 hypothesised causal bases of Alzheimer's disease.
Tyler SEB, Tyler LDK. Tyler SEB, et al. Nat Prod Bioprospect. 2022 Aug 23;12(1):34. doi: 10.1007/s13659-022-00354-z. Nat Prod Bioprospect. 2022. PMID: 35996065 Free PMC article. - Rivastigmine lowers Aβ and increases sAPPα levels, which parallel elevated synaptic markers and metabolic activity in degenerating primary rat neurons.
Bailey JA, Ray B, Greig NH, Lahiri DK. Bailey JA, et al. PLoS One. 2011;6(7):e21954. doi: 10.1371/journal.pone.0021954. Epub 2011 Jul 22. PLoS One. 2011. PMID: 21799757 Free PMC article.
References
- Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42.
- Alzheimer's Association 2009 Alzheimer's disease facts and figures. Alzheimers. Dement. 2009;5:234–270. - PubMed
- Bailey JA, Lahiri DK. Neuronal differentiation is accompanied by increased levels of SNAP-25 protein in fetal rat primary cortical neurons: implications in neuronal plasticity and Alzheimer's disease. Ann. N. Y. Acad. Sci. 2006;1086:54–65. - PubMed
- Bailey JA, Lahiri DK. P2-317: Rivastigmine decreases amyloid-β precursor protein and increases synaptic markers in primary neuronal cultures. 2008. p. T465.
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993;35:567–576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG18379/AG/NIA NIH HHS/United States
- R01 AG018884/AG/NIA NIH HHS/United States
- R01 AG018884-07/AG/NIA NIH HHS/United States
- R01 AG018379/AG/NIA NIH HHS/United States
- AG18884/AG/NIA NIH HHS/United States
- R01 AG018379-10/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical