Alveolar macrophages in allergic asthma: an expression signature characterized by heat shock protein pathways - PubMed (original) (raw)
Alveolar macrophages in allergic asthma: an expression signature characterized by heat shock protein pathways
Anne-Marie Madore et al. Hum Immunol. 2010 Feb.
Abstract
The implication of alveolar macrophages (AM) in asthma, a T(h)2 disease, has not been well characterized. Thus, the goal of this study is to better characterize AM phenotype of allergic asthmatic compared with normal subjects using genomic expression analyses. Microarray analyses were performed with AM isolated from bronchoalveolar lavage. Robust multiarray analysis (RMA) normalization and Smyth's moderated t test were used to select differentially expressed genes. Fifty differentially expressed genes were identified. Nineteen have been classified in categories linked to stress or immune responses and among them; nine are part of the heat shock protein (HSP) family. Difference of expression for three (HSPD1, PRNP, SERPINH1) of the five selected genes were validated using real-time reverse transcription-polymerase chain reaction. Enzyme-linked immunosorbent assay was used to measure the protein level of heat shock protein 60 (HSP60), the protein encoded by HSPD1, and showed difference in AM protein level between allergic asthmatic and control subjects. In summary, this study suggests that HSP gene family, particularly HSP60, is involved in AM functions in a context of allergic asthma. These results also support the involvement of AM immune functions in the development of an allergic asthmatic response.
Copyright 2010 American Society for Histocompatibility and Immunogenetics. Published by Elsevier Inc. All rights reserved.
Figures
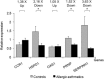
Fig. 1
Expression of the five selected genes using real-time RT-PCR. Chemokine (C-C motif) receptor 1 (CCR1), heat shock 60-kDa protein 1 (HSPD1), 2,5-oligoadenylate synthetase 1 (40–46 kDa) (OAS1), prion protein (PRNP) and serpin peptidase inhibitor, clade H (heat shock protein 47), member 1 (SERPINH1) mRNA expression by alveolar macrophages (AM) isolated from bronchoalveolar lavage of control (gray bars) and allergic asthmatic (black bars) subjects. Measure of the mRNA expression by real-time RT-PCR was done in triplicate with negative control and normalized to GAPDH expression using two-standard curves method (n = 4). Data are expressed as mean ± SEM values. HSPD1, PRNP, and SERPINH1 mRNA are significantly (*p < 0.05) underexpressed in AM of asthmatic subjects compared with controls.
Fig. 2
HSP60 protein expression by alveolar macrophages of control and allergic asthmatic subjects. Bronchoalveolar cells of four control subjects and four allergic asthmatic subjects were fixed with PLP-sucrose and immuncytochemistry was done using ABComplex/AP method and Fast-Red coloration with Mayer's hematoxylin counterstaining (magnification ×1,000). Immunocytochemistry for HSP60 expression of (A) isotypic negative control, (B) control subjects, and (C) allergic asthmatic subjects. Results shown are representative. There was no difference in positive alveolar macrophage cell count for HSP60 between control and asthmatic subjects (90% ± 5% and 90 ± 4% of positive cells for control and asthmatic subjects, respectively).
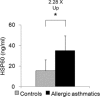
Fig. 3
Protein level of HSP60 in alveolar macrophages (AM) of control and allergic asthmatic subjects. AM were purified from bronchoalveolar lavage and lysed to measure HSP60 by ELISA. Protein levels for control (gray bars) and allergic asthmatic (black bars) subjects are shown (n = 5). Data are expressed as mean ± SEM values. Level of HSP60 is significantly higher (*p < 0.05) in AM of asthmatic subjects compared with controls.
Similar articles
- Alveolar macrophage-derived vascular endothelial growth factor contributes to allergic airway inflammation in a mouse asthma model.
Song C, Ma H, Yao C, Tao X, Gan H. Song C, et al. Scand J Immunol. 2012 Jun;75(6):599-605. doi: 10.1111/j.1365-3083.2012.02693.x. Scand J Immunol. 2012. PMID: 22324377 - A comparison of two sets of microarray experiments to define allergic asthma expression pattern.
Chamberland A, Madore AM, Tremblay K, Laviolette M, Laprise C. Chamberland A, et al. Exp Lung Res. 2009 Jun;35(5):399-410. doi: 10.1080/01902140902745174. Exp Lung Res. 2009. PMID: 19842841 - Gene expression profiling in allergy and asthma.
Schmidt-Weber CB. Schmidt-Weber CB. Chem Immunol Allergy. 2006;91:188-94. doi: 10.1159/000090281. Chem Immunol Allergy. 2006. PMID: 16354959 Review. - MicroRNA regulation of allergic inflammation and asthma.
Pua HH, Ansel KM. Pua HH, et al. Curr Opin Immunol. 2015 Oct;36:101-8. doi: 10.1016/j.coi.2015.07.006. Epub 2015 Aug 5. Curr Opin Immunol. 2015. PMID: 26253882 Free PMC article. Review.
Cited by
- The Utility of Resolving Asthma Molecular Signatures Using Tissue-Specific Transcriptome Data.
Ghosh D, Ding L, Bernstein JA, Mersha TB. Ghosh D, et al. G3 (Bethesda). 2020 Nov 5;10(11):4049-4062. doi: 10.1534/g3.120.401718. G3 (Bethesda). 2020. PMID: 32900903 Free PMC article. - Role of Human Macrophage Polarization in Inflammation during Infectious Diseases.
Atri C, Guerfali FZ, Laouini D. Atri C, et al. Int J Mol Sci. 2018 Jun 19;19(6):1801. doi: 10.3390/ijms19061801. Int J Mol Sci. 2018. PMID: 29921749 Free PMC article. Review. - The role of macrophage subtypes and exosomes in immunomodulation.
Gharavi AT, Hanjani NA, Movahed E, Doroudian M. Gharavi AT, et al. Cell Mol Biol Lett. 2022 Oct 3;27(1):83. doi: 10.1186/s11658-022-00384-y. Cell Mol Biol Lett. 2022. PMID: 36192691 Free PMC article. Review. - Sex differences in the response of the alveolar macrophage proteome to treatment with exogenous surfactant protein-A.
Phelps DS, Umstead TM, Floros J. Phelps DS, et al. Proteome Sci. 2012 Jul 23;10(1):44. doi: 10.1186/1477-5956-10-44. Proteome Sci. 2012. PMID: 22824420 Free PMC article. - GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation.
Das S, Miller M, Beppu AK, Mueller J, McGeough MD, Vuong C, Karta MR, Rosenthal P, Chouiali F, Doherty TA, Kurten RC, Hamid Q, Hoffman HM, Broide DH. Das S, et al. Proc Natl Acad Sci U S A. 2016 Nov 15;113(46):13132-13137. doi: 10.1073/pnas.1610433113. Epub 2016 Oct 31. Proc Natl Acad Sci U S A. 2016. PMID: 27799535 Free PMC article.
References
- National Institute of Health . US Department Health Human Services, National Heart, Lung and Blood Institute; Washington, DC: 1997. Guidelines for the Diagnosis and Management of Asthma: Report Numbers 97–4,051.
- Thepen T., Kraal G., Holt P.G. The role of alveolar macrophages in regulation of lung inflammation. Ann NY Acad Sci. 1994;725:200–206. - PubMed
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous