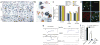Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes - PubMed (original) (raw)
Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes
Juan E Belforte et al. Nat Neurosci. 2010 Jan.
Abstract
Cortical GABAergic dysfunction may underlie the pathophysiology of psychiatric disorders, including schizophrenia. Here, we characterized a mouse strain in which the essential NR1 subunit of the NMDA receptor (NMDAR) was selectively eliminated in 40-50% of cortical and hippocampal interneurons in early postnatal development. Consistent with the NMDAR hypofunction theory of schizophrenia, distinct schizophrenia-related symptoms emerged after adolescence, including novelty-induced hyperlocomotion, mating and nest-building deficits, as well as anhedonia-like and anxiety-like behaviors. Many of these behaviors were exacerbated by social isolation stress. Social memory, spatial working memory and prepulse inhibition were also impaired. Reduced expression of glutamic acid decarboxylase 67 and parvalbumin was accompanied by disinhibition of cortical excitatory neurons and reduced neuronal synchrony. Postadolescent deletion of NR1 did not result in such abnormalities. These findings suggest that early postnatal inhibition of NMDAR activity in corticolimbic GABAergic interneurons contributes to the pathophysiology of schizophrenia-related disorders.
Figures
Figure 1
Generation of a corticolimbic GABAergic neuron-restricted Cre line. (a) Endogenous Ppp1r2 immunoreactivity in primary somatosensory cortex (S1) of a 4-week-old wild-type C57BL/6N mouse (Alexa 488, green) colocalized with antibody to GAD67 (Cy3, red). (b) Ppp1r2_-c_re mice (8-week-old) crossed with the _loxP_-flanked Rosa26-lacZ reporter. Cre recombination (β-galactosidase (β-gal)–Cy3, red) occurred in Ppp1r2-positive neurons (Alexa 488, green) in S1. (c–e) Spatial distribution of Cre recombinase activity in parasagittal sections from an 8-week-old Ppp1r2-cre; Rosa26-lacZ double-transgenic mouse stained with X-Gal (blue) and Safranin O (red). Sections are from S1 cortex (c), prefrontal cortex (d) and hippocampus (e). cc, corpus callosum, fim, fimbria; olf, olfactory bulb. (f– i) Immunofluorescence of coronal sections from Ppp1r2-Cre; Rosa26-lacZ mice. High-magnification confocal images were used to quantify colocalization. More than 92% of Cre-targeted neurons (β-gal positive) expressed GAD67 (f) but not TBR1 (g). Approximately 75% of GAD67-positive neurons were parvalbumin (PV) positive (h) and none were calretinin (CR) positive (i). Lower left insets, higher magnification of boxed regions. Scale bars represent 100 μm (a,b,f–i) and 500 μm (c–e).
Figure 2
Restricted NR1 deletion in GABAergic neurons in cortex and hippocampus. (a) Representative photomicrographs of nonradioisotope double in situ hybridization of parasagittal sections of S1 from 4-week-old Flox-A (_NR1loxP/loxP_-line A) and mutant (Ppp1r2-cre+/−; _NR1loxP/loxP_-line A, KO) mice (GAD67, red; NR1 blue; colocalization, brown). Scale bars represent 50 μm. (b) High magnification of boxed area in the mutant section depicting GABAergic neurons with (brown arrows) and without (red arrows) detectable levels of NR1 mRNA. Non-GABAergic neurons expressing NR1 mRNA are indicated by blue arrows. Scale bar represents 20 μm. (c) Regional and temporal quantification of NR1 mRNA in GAD67-positive neurons. BLA, basolateral amygdala; DG, dentate gyrus; mPFC, medial prefrontal cortex. n = 3 for each mutant age, controls n = 4 at 4–20 weeks old. (d) Immunohistochemical characterization of a neuron filled with biocytin (blue) during whole-cell patch-clamp recording in S1 slices from Ppp1r2-cre mice crossed with the _loxP_-flanked Rosa26-EYFP mice. Cre-targeted neurons were identified by EYFP expression during recording and later stained for GFP (green). The white arrow indicates triple colocalization of EYFP, biocytin and parvalbumin (red). (e) Representative traces of whole-cell patch-clamp recordings from Cre-targeted neurons in S1 slices of Cre control (Ppp1r2-cre+/−; Rosa26-EYFPloxP/+) and mutant (Ppp1r2-cre+/−; _NR1loxP/loxP_-line A; Rosa26-EYFPloxP/+, KO) mice under different pharmacological conditions (black traces; black scale bars indicate 20 pA and 500 ms). Spontaneous EPSCs (arrowheads) recorded in the presence of NBQX in the Cre controls, but not in mutant mice, were reduced by the NMDA channel blocker AP5. Blue traces indicate the average of 20 sEPSCs; blue scale bars represent 20 pA and 50 ms. The recording of Cre control is from the neuron with the white arrow in d. (f) sEPSC amplitudes of Cre-targeted S1 neurons under baseline conditions, AMPA blockade (+NBQX), and AMPA/NMDA blockade (+NBQX/+APV). We observed no NMDA components in the mutant mice. Mann-Whitney U Test, * P < 0.05 versus Cre control. Data are mean ± s.e.m.; number of cells is indicated in parentheses from KO (n = 11) and Cre control (n = 10).
Figure 3
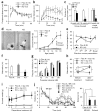
Early postnatal NR1 deletion leads to schizophrenia-related behaviors. (a,b) Increased peripheral locomotion following exposure to a novel open field and decreased exploration of the anxiogenic central area in mutant mice versus controls (Cre and Flox-A) (repeated measures ANOVA/LSD post hoc test, * P < 0.05 versus Flox-A and Cre). (c) Social isolation induced anxiety-like behavior in the elevated plus maze, as shown by the decreased number of entries into unprotected open arms by mutant mice compared with controls (ANOVA/LSD post hoc test, * P < 0.05 versus controls). (d) Left, representative pictures of the nests of Flox-A and mutant mice (arrow, unused material). Right, single-housed mutant mice displayed a significant deficit in nest construction starting at 14 weeks of age, as determined by weighing the unused nestlet material after overnight housing in a new cage (two-way ANOVA/LSD post hoc test, * P < 0.05 versus Flox-A and Cre). Group-housed mutant mice had a smaller deficit compared with age-matched single-housed mutants (16 weeks old, ANOVA/LSD post hoc test, # P < 0.05 versus controls and single-housed mutant mice). (e) The social-recognition test revealed impairment in social short-term memory in the mutant. Flox-A control mice decreased social investigation time following repeated exposures (1 min each) to a stimulus mouse. A fifth dishabituation trial elicited an increased response to a novel mouse, showing individual recognition. In contrast, mutant mice did not show habituation after repeated presentations of the stimulus mouse (second to fourth presentation, repeated measures ANOVA/LSD post hoc test, * P < 0.05 versus Flox-A), suggesting social amnesia, and reduced investigation time during the first presentation. (f) In the Y-maze spontaneous alternation task, the mutant mice alternated between the arms at the chance level (dotted line; ANOVA/LSD post hoc test, * P < 0.005 versus Flox-A and Cre). (g) Mutant mice at 10–12 weeks of age were impaired in prepulse inhibition of the auditory startle reflex across prepulse intensities (two-way ANOVA/LSD post hoc test, * P < 0.005 for genotype factor). (h) Chronic treatment with risperidone (2.5 mg per kg of body weight per d for 3 weeks in drinking water) ameliorated the working memory deficit of the mutant mice in the Y-maze spontaneous alternation task (repeated-measures ANOVA/LSD post hoc test, * P < 0.05, versus. Flox-A, # P < 0.001 mutant mice treated with risperidone versus solvent). Dotted line indicates chance level. (i) Chronic risperidone treatment did not rescue the deficit in social nest building (repeated-measures ANOVA/LSD post hoc test, P = 0.56 mutant mice treated with risperidone versus solvent, P < 0.01 mutant mice treated with risperidone versus Flox-A treated with risperidone). (j) Noncompetitive NMDAR antagonist–induced locomotion was diminished in mutant mice. Left, time course of MK-801–induced locomotor activity (broken line, MK-801 injection, 0.2 mg per kg intraperitoneal; repeated-measures ANOVA/LSD post hoc test, * P < 0.05 versus MK801-treated mutant mice (MK), # P < 0.05 versus mutant mice treated with solvent). Right, cumulative distance traveled after MK-801 treatment (30–120 min; one-way ANOVA/LSD post hoc test, * P < 0.02). Data are mean ± s.e.m.; n is indicated in parentheses or plot bars. Cre, Ppp1r2-cre+/−; Flox-A, _NR1loxP/loxP_-line A; KO, Ppp1r2-cre+/−; _NR1loxP/loxP_-line A.
Figure 4
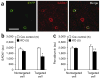
Decreased expression of GAD67 and parvalbumin in NR1-deleted neurons. (a) Confocal photomicrographs of coronal sections (layer II/III S1) immunostained with antibodies to GFP (green, Alexa488) and GAD67 (red, Cy3) to identify Cre-targeted neurons from mutant or Ppp1r2-cre mice crossed with _loxP_-flanked Rosa26-EYFP mice (12 weeks old). Scale bar represents 40 μm. (b,c) Quantitative analysis of GAD67 (b) and parvalbumin (c) immunoreactivity in Cre-targeted and nontargeted neurons in S1 coronal sections from Cre controls (Ppp1r2-cre+/−; Rosa26-EYFPloxP/+) and mutant mice (_Ppp1r2-cre+/−; NR1loxP/loxP_-line A; Rosa26-EYFPloxP/+) at 12–14 weeks old). Bars depict mean ± s.e.m., animal number is indicated in parantheses, two-way ANOVA/LSD post hoc test, * P < 0.005, ** P < 0.01.
Figure 5
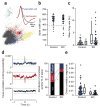
Increased firing of cortical excitatory neurons accompanied by reduced neuronal synchrony. (a) A representative cluster analysis of spike waveforms, sorted into individual units on the basis of spike amplitude distribution. Inset, average spike shape for putative pyramidal neuron (blue) and interneuron (red). Note the characteristic difference in the slope of the afterhyperpolarization phase. (b) No difference in spike-width distributions in neurons recorded from S1 in 13–14 week-old freely moving mutant mice (Ppp1r2-cre+/−; _NR1loxP/loxP_-line A, n = 4, KO) and controls (n = 2 for Cre (Ppp1r2-cre+/−), n = 2 for Flox-A (_NR1loxP/loxP_-line A)) during exploration of an unfamiliar linear track. Units with spike widths >380 μs were considered to be putative pyramidal neurons. (c) Mean firing rates of putative S1 pyramidal neurons were significantly higher in mutant mice compared with controls. Each dot is the mean firing rate for an individual neuron (n = 34 control, n = 29 mutant mice). Box plots depict medians (box centers), interquartile ranges (box boundaries) and 10–90th percentiles (whiskers). Mann-Whitney U test, * P < 0.005. (d) Synchronized activity of pairs of nearby neurons from the recording shown in c was assessed by computing the cross-correlation for all pairs of pyramidal neurons recorded from a single tetrode. Representative cross-correlograms from controls exemplifying three different correlation responses: significantly positive (blue), significantly negative (red) and nonsignificant cross-correlation (black). Dotted lines indicate 99% confidence interval. Mutants showed a higher incidence of nonsignificantly correlated pairs of neurons (controls, 26 of 62 pairs (42%); mutant mice s, 31 of 42 pairs (74%); χ2 test, * P < 0.005]. (e) Decrease in the magnitude of cross-correlations across all pairs in mutant mice regardless of the polarity. Each dot represents an individual pair. Mann-Whitney U test, * P < 0.0005.
Figure 6
No schizophrenia-related phenotypes were observed following adult NR1 deletion. (a) Left, representative photomicrographs from cortex of the adult knockout mutant (_Ppp1r2-cre+/−; NR1loxP/loxP_-line B) prior to onset of recombination (8 weeks old) and after recombination was completed (20 weeks old). Brown arrows indicate colocalization of Gad67 and NR1 mRNAs, and red arrows indicate GAD67-positive neurons lacking NR1 mRNA. Right, quantification of double in situ hybridization detection of NR1 and Gad67 mRNA in the adult knockout mutants (n = 3 for each age). (b,c) Single-housed adult knockout mutants tested at 25–29 weeks of age were not impaired in a social-recognition test (b) or PPI (c). (d) After crossing with the _loxP_-flanked Rosa26-EYFP mice, adult knockout mutants (Ppp1r2-cre+/−; _NR1loxP/loxP_-line B; Rosa26-EYFPloxP/+) showed no significant change in GAD67 or parvalbumin immunoreactivity in Cre-targeted neurons of layer II/III S1 compared to Cre controls (Ppp1r2-cre+/−; Rosa26-EYFPloxP/+) at 26–27 weeks old. T, Cre-targeted neurons; Non-T, nontargeted neurons. Data are mean ± s.e.m.; n is indicated in parentheses.
Figure 7
Adult NR1 deletion did not alter firing rate or synchronous firing of cortical excitatory neurons. (a) We found no difference in the mean firing rates of putative pyramidal neurons from S1 between 25–28-week-old controls (n = 2 for Cre (Ppp1r2-cre+/−), n = 2 for Flox-B (_NR1loxP/loxP_-line B)) and adult knockout mutants (Ppp1r2-cre+/−; _NR1loxP/loxP_-line B, (n = 4) during exploration of an unfamiliar linear track. Each dot represents the mean firing rate of an individual neuron (n = 31 for control, n = 33 for mutant). Mann-Whitney U test, P = 0.87. (b) Synchronized activity of pairs of nearby neurons from the recording shown in a. No difference was observed in the distribution of cross-correlation patterns between adult knockout mutants and age-matched controls (χ2 test, P = 0.24). (c) There was no difference in the magnitude of cross-correlations across all pairs between groups (Mann-Whitney U test, P = 0.32). Box plots depict medians (box centers), interquartile ranges (box boundaries) and 10–90th percentiles (whiskers). Each dot represents an individual pair.
Comment in
- Testing the glutamate hypothesis of schizophrenia.
Gordon JA. Gordon JA. Nat Neurosci. 2010 Jan;13(1):2-4. doi: 10.1038/nn0110-2. Nat Neurosci. 2010. PMID: 20033077 No abstract available.
Similar articles
- Cortical parvalbumin GABAergic deficits with α7 nicotinic acetylcholine receptor deletion: implications for schizophrenia.
Lin H, Hsu FC, Baumann BH, Coulter DA, Anderson SA, Lynch DR. Lin H, et al. Mol Cell Neurosci. 2014 Jul;61:163-75. doi: 10.1016/j.mcn.2014.06.007. Epub 2014 Jun 28. Mol Cell Neurosci. 2014. PMID: 24983521 Free PMC article. - Social isolation exacerbates schizophrenia-like phenotypes via oxidative stress in cortical interneurons.
Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K. Jiang Z, et al. Biol Psychiatry. 2013 May 15;73(10):1024-34. doi: 10.1016/j.biopsych.2012.12.004. Epub 2013 Jan 21. Biol Psychiatry. 2013. PMID: 23348010 Free PMC article. - Interneuron NMDA Receptor Ablation Induces Hippocampus-Prefrontal Cortex Functional Hypoconnectivity after Adolescence in a Mouse Model of Schizophrenia.
Alvarez RJ, Pafundo DE, Zold CL, Belforte JE. Alvarez RJ, et al. J Neurosci. 2020 Apr 15;40(16):3304-3317. doi: 10.1523/JNEUROSCI.1897-19.2020. Epub 2020 Mar 23. J Neurosci. 2020. PMID: 32205341 Free PMC article. - The origin of NMDA receptor hypofunction in schizophrenia.
Nakazawa K, Sapkota K. Nakazawa K, et al. Pharmacol Ther. 2020 Jan;205:107426. doi: 10.1016/j.pharmthera.2019.107426. Epub 2019 Oct 16. Pharmacol Ther. 2020. PMID: 31629007 Free PMC article. Review. - GABAergic interneuron origin of schizophrenia pathophysiology.
Nakazawa K, Zsiros V, Jiang Z, Nakao K, Kolata S, Zhang S, Belforte JE. Nakazawa K, et al. Neuropharmacology. 2012 Mar;62(3):1574-83. doi: 10.1016/j.neuropharm.2011.01.022. Epub 2011 Jan 26. Neuropharmacology. 2012. PMID: 21277876 Free PMC article. Review.
Cited by
- Dalzanemdor (SAGE-718), a novel, investigational N-methyl-D-aspartate receptor positive allosteric modulator: Safety, tolerability, and clinical pharmacology in randomized dose-finding studies in healthy participants and an open-label study in participants with Huntington's disease.
Koenig A, Lewis M, Wald J, Li S, Varoglu M, Dai J, Sankoh A, Paumier K, Doherty J, Quirk M. Koenig A, et al. Clin Transl Sci. 2024 Jul;17(7):e13852. doi: 10.1111/cts.13852. Clin Transl Sci. 2024. PMID: 38988035 Free PMC article. Clinical Trial. - Serine racemase deletion alters adolescent social behavior and whole-brain cFos activation.
Brown SE, Wang ZZ, Newman EL, Engin E, Berretta S, Balu DT, Folorunso OO. Brown SE, et al. Front Psychiatry. 2024 Jun 24;15:1365231. doi: 10.3389/fpsyt.2024.1365231. eCollection 2024. Front Psychiatry. 2024. PMID: 38979499 Free PMC article. - GluK1 kainate receptors are necessary for functional maturation of parvalbumin interneurons regulating amygdala circuit function.
Haikonen J, Szrinivasan R, Ojanen S, Rhee JK, Ryazantseva M, Sulku J, Zumaraite G, Lauri SE. Haikonen J, et al. Mol Psychiatry. 2024 Jun 28. doi: 10.1038/s41380-024-02641-2. Online ahead of print. Mol Psychiatry. 2024. PMID: 38942774 - The Pathophysiological Underpinnings of Gamma-Band Alterations in Psychiatric Disorders.
Palmisano A, Pandit S, Smeralda CL, Demchenko I, Rossi S, Battelli L, Rivolta D, Bhat V, Santarnecchi E. Palmisano A, et al. Life (Basel). 2024 Apr 30;14(5):578. doi: 10.3390/life14050578. Life (Basel). 2024. PMID: 38792599 Free PMC article. Review. - Parvalbumin interneuron deficits in schizophrenia.
Marín O. Marín O. Eur Neuropsychopharmacol. 2024 May;82:44-52. doi: 10.1016/j.euroneuro.2024.02.010. Epub 2024 Mar 14. Eur Neuropsychopharmacol. 2024. PMID: 38490084 Free PMC article. Review.
References
- Lodge D, Anis NA. Effects of phencyclidine on excitatory amino acid activation of spinal interneurones in the cat. Eur J Pharmacol. 1982;77:203–204. - PubMed
- Javitt DC. Negative schizophrenic symptomatology and the PCP (phencyclidine) model of schizophrenia. Hillside J Clin Psychiatry. 1987;9:12–35. - PubMed
- Olney JW. In: Excitatory Acid Acids in Health and Disease. Lodge D, editor. Wiley; London: 1988. pp. 337–351.
- Deutsch SI, Mastropaolo J, Schwartz BL, Rosse RB, Morihisa JMA. “glutamatergic hypothesis” of schizophrenia. Rationale for pharmacotherapy with glycine. Clin Neuropharmacol. 1989;12:1–13. - PubMed
- Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials