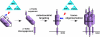Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis - PubMed (original) (raw)
Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis
Hyungjin Kim et al. Mol Cell. 2009.
Abstract
While activation of BAX/BAK by BH3-only molecules (BH3s) is essential for mitochondrial apoptosis, the underlying mechanisms remain unsettled. Here we demonstrate that BAX undergoes stepwise structural reorganization leading to mitochondrial targeting and homo-oligomerization. The alpha1 helix of BAX keeps the alpha9 helix engaged in the dimerization pocket, rendering BAX as a monomer in cytosol. The activator BH3s, tBID/BIM/PUMA, attack and expose the alpha1 helix of BAX, resulting in secondary disengagement of the alpha9 helix and thereby mitochondrial insertion. Activator BH3s remain associated with the N-terminally exposed BAX through the BH1 domain to drive homo-oligomerization. BAK, an integral mitochondrial membrane protein, has bypassed the first activation step, explaining why its killing kinetics are faster than those of BAX. Furthermore, death signals initiated at ER induce BIM and PUMA to activate mitochondrial apoptosis. Accordingly, deficiency of Bim/Puma impedes ER stress-induced BAX/BAK activation and apoptosis. Our study provides mechanistic insights regarding the spatiotemporal execution of BAX/BAK-governed cell death.
Figures
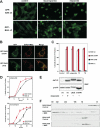
Figure 1. Mitochondrial targeting and homo-oligomerization are two separable, essential steps of BAX activation
(A) C-terminal α9 helix targets BAX to the mitochondria. Fluorescence microscopy of Bax/Bak DKO MEFs reconstituted with GFP-BAX or GFP-BAXΔC before or after treatment with staurosporine (12 hr) or etoposide (15 hr). (B) A single amino acid substitution at the BH3 domain or α9 helix of BAX constitutively targets BAX to the mitochondria. Fluorescence microscopy of DKO MEFs stably reconstituted with GFP-BAX S184V or L63E followed by retroviral transduction of DsRed-Mito. (C) Mitochondrially localized BAX is not constitutively active. DKO MEFs reconstituted with wt or mutant BAX were treated with staurosporine (18 hr), etoposide (18 hr), tunicamycin (TC, 30 hr), or thapsigargin (TG, 30 hr) to induce apoptosis. (D) BAX S184V displays faster proapoptotic kinetics than wt BAX. DKO MEFs reconstituted with wt BAX or BAX S184V were treated with etoposide or tunicamycin for the indicated time. Asterisk, P<0.05. (E) Mitochondrially localized BAX mutants or activated BAX expose the N-terminal α1 helix. DKO MEFs reconstituted with wt BAX before or after treatment with etoposide for 15 hr, or reconstituted with indicated BAX mutants were lysed in 1% CHAPS and then immunoprecipitated with the 6A7 antibody. Immunoprecipitates were analyzed by anti-BAX (N20) immunoblots. (F) BH3 domain is required for the homo-oligomerization of BAX. Mitochondria isolated from DKO MEFs reconstituted with BAX S184V or BAX L63E were incubated with recombinant tBID (1 ng/μl) and solubilized in 2 % CHAPS buffer. Protein lysates (200 μg) were subjected to Superdex 200 (HR 10/30) gel filtration chromatography and fractions were analyzed by anti-BAX immunoblots. Data shown in (C) and (D) are mean ± s.d. from three independent experiments. Cell death was quantified by Annexin-V.
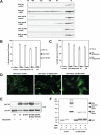
Figure 2. Characterization of homo-oligomerization and mitochondrial targeting of BAX
(A) BH1 and BH3 domain mutants of BAX fail to undergo homo-oligomerization in response to tBID. Mitochondria isolated from DKO MEFs reconstituted with wt or mutant BAX were treated with recombinant tBID (1 ng/μl) and solubilized in 2 % CHAPS buffer. Protein lysates (200 μg) were subjected to Superdex 200 (HR 10/30) gel filtration chromatography and fractions were analyzed by anti-BAX immunoblots. (B and C) BH1 and BH3 domain mutants of BAX fail to trigger apoptosis. DKO MEFs reconstituted with wt or mutant BAX were treated with staurosporine (18 hr), etoposide (18 hr), tunicamycin (30 hr) or thapsigargin (30 hr) to induce apoptosis. Cell death was quantified by Annexin-V. Data shown are mean ± s.d. from three independent experiments. (D) S184V mutation fully targets BAX G108E but only partially targets BAX G67R to the mitochondria. Fluorescence microscopy of DKO MEFs reconstituted with the indicated GFP-tagged BAX mutants. (E) The N-terminal exposure of BAX correlates with mitochondrial targeting. DKO MEFs reconstituted with wt BAX before or after treatment with etoposide (15 hr), or reconstituted with indicated BAX mutants were lysed in 1% CHAPS and then immunoprecipitated with the 6A7 antibody. Immunoprecipitates were analyzed by anti-BAX (N20) immunoblots. (F) BH1 domain is required for the homo-oligomerization of BAX. Mitochondria isolated from DKO MEFs reconstituted with BAX S184V or BAX G108E/S184V were incubated with recombinant tBID for 30 min and then treated with BMH crosslinker. The BAX oligomers were detected by an anti-BAX immunoblot.
Figure 3. BH1 and BH3 domains of BAX are required for its activation
(A) BH1 and BH3 domain mutants of BAX fail to translocate to mitochondria upon apoptotic signals. Fluorescence microscopy of DKO MEFs reconstituted with GFP-tagged wt or mutant BAX before or after treatment with staurosporine (12 hr) or etoposide (15 hr). (B) BH1 and BH3 domain mutants of BAX fail to expose the α1 helix in response to DNA damage or tBID. DKO MEFs reconstituted with wt or mutant BAX were untreated, treated with etoposide, or transduced with tBID by retrovirus. Cells lysed in 1 % CHAPS were subject to the 6A7 immunoprecipitation, followed by an anti-BAX (N20) immunoblot. (C) BH1 and BH3 domain mutants of BAX fail to insert into the MOM in response to tBID. Mitochondria isolated from DKO MEFs reconstituted wt or mutant BAX were mock treated or treated with IVTT tBID, followed by alkaline extraction. The alkali-sensitive supernatant (S) and alkali-resistant pellet (P) fractions were analyzed by anti-BAX immunoblots. The numbers shown denote the percent of BAX quantified by densitometry. (D) BIM and PUMA induce the mitochondrial insertion of BAX. Mitochondria isolated from DKO MEFs reconstituted with wt BAX were mock treated or treated with IVTT BIM or PUMA, followed by alkali extraction. The alkali-sensitive supernatant (S) and alkali-resistant pellet (P) fractions were analyzed by anti-BAX immunoblots. The numbers shown denote the percent of BAX quantified by densitometry.
Figure 4. Activation of BAX can be dissected into two sequential steps, mitochondrial targeting and homo-oligomerization, both of which require activator BH3s
(A) The α1 helix of BAX keeps the α9 helix engaged in the dimerization pocket. Radiolabeled IVTT HA-tagged BAXΔC or HA-tagged BAXΔNΔC in combination with GST-α9 or GST-α9 S184V were subjected to anti-HA immunoprecipitation in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography. Asterisk denotes the degradation products. (B) The L63E mutation in BAX disrupts the binding of the α1 helix to the rest of the protein, resulting in N-terminal exposure and mitochondrial targeting. Radiolabeled IVTT HA3-tagged BAX α1 helix in combination with BAXΔN wt, L63E or G67R were subjected to anti-HA immunoprecipitation in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography. (C) The S184V mutation in BAX destabilizes the binding of the α1 helix to the rest of the protein. Radiolabeled IVTT HA3-tagged BAX α1 helix in combination with BAXΔN wt or S184V were subjected to anti-HA immunoprecipitation in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography. (D) tBID, BIM, and PUMA bind to the α1 helix of BAX. Radiolabeled IVTT HA3-tagged BAX α1 helix in combination with tBID, BIM, or PUMA were subjected to anti-HA immunoprecipitation in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography. (E) tBID, BIM, and PUMA, but not BAD, induce the N-terminal exposure of BAX and remain associated with the N-terminally exposed BAX. Radiolabeled IVTT BAX incubated with tBID, BIM, PUMA, or BAD were immunoprecipitated with the 6A7 antibody in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography. (F) tBID, BIM, and PUMA induce the homo-oligomerization of BAX S184V. Mitochondria isolated from DKO MEFs reconstituted with BAX S184V were incubated with IVTT tBID, BIM, or PUMA for 30 min and then treated with BMH crosslinker. The BAX oligomers were detected by an anti-BAX immunoblot. (G) BH1 domain is required for N-terminally exposed BAX to interact with tBID. Radiolabeled IVTT BAX L63E or BAX L63E/G108E incubated with tBID were immunoprecipitated with the 6A7 antibody in 1 % CHAPS. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography.
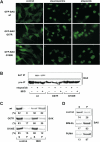
Figure 5. BH1 and BH3 domains are required for the homo-oligomerization and proapoptotic activity of BAK
(A) BH1 and BH3 domain mutants of BAK fail to undergo homo-oligomerization in response to tBID. Mitochondria isolated from DKO MEFs reconstituted with wt or mutant BAK were treated with recombinant tBID (1 ng/μl) and solubilized in 2 % CHAPS buffer. Protein lysates (200 μg) were subjected to Superdex 200 (HR 10/30) gel filtration chromatography and fractions were analyzed by anti-BAK immunoblots. (B) BH1 and BH3 domain mutants of BAK fail to form homo-dimers. Radiolabeled IVTT N-terminal HA3-tagged wt or mutant BAK plus non-tagged counterparts were immunoprecipitated with anti-HA antibody. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography. Asterisk denotes degradation products. (C) BH1 and BH3 domain mutants of BAK fail to trigger apoptosis. DKO MEFs reconstituted with wt or mutant BAK were treated with staurosporine (18 hr), etoposide (18 hr), tunicamycin (30 hr) or thapsigargin (30 hr) to induce apoptosis. Cell death was quantified by Annexin-V. Data shown are mean ± s.d. from three independent experiments. (D) BH1 and BH3 domain mutants of BAK are localized at the mitochondria. Fluorescence microscopy of DKO MEFs stably reconstituted with YFP-tagged wt or mutant BAK followed by retroviral transduction of DsRed-Mito. (E) BH1 domain is required for BAK to interact with tBID. Radiolabeled IVTT BAK wt, BAK BH3 mt (L75E) or BH1 mt (W122A/G123E/R124A) incubated with tBID-HA were immunoprecipitated with anti-HA antibody. Immunoprecipitates and pre-IP input were analyzed by Nu-PAGE and autoradiography. (F) BAK constitutively exposes its N-terminal α1 helix. Mitochondria isolated from wt MEFs, mock treated or treated with tBID, were lysed in 1% CHAPS and then immunoprecipitated with the α1 helix specific anti-BAK antibody (NT). Immunoprecipitates and pre-IP input were analyzed by anti-BAK (G23) immunoblots. Mitochondria isolated from DKO MEFs serve as a negative control. (G) BAK constitutively exposes its N-terminal α1 helix. 4T1 or N18 cells before or after staurosporine treatment were lysed in 1% CHAPS and then immunoprecipitated with the α1 helix specific anti-BAK antibody (NT). Immunoprecipitates and pre-IP input were analyzed by anti-BAK (G23) immunoblots.
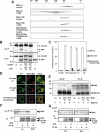
Figure 6. BIM and PUMA activate mitochondrial BAK and BAX to execute apoptosis upon ER stress
(A) Schematic of the mitochondria- and ER-targeted BAK chimera mutants. (B) Mitochondria- but not ER-targeted BAK is required for apoptosis triggered by intrinsic death signals. DKO MEFs reconstituted with wt or mutant BAK were treated with staurosporine (9 hr), etoposide (18 hr), tunicamycin (30 hr) or thapsigargin (30 hr) to induce apoptosis. (C) BIM and PUMA are induced by ER stress. DKO MEFs untreated or treated with tunicamycin or thapsigargin for 18 hr were lysed and analyzed by anti-BIM and PUMA immunoblots. (D) BIM and PUMA are required for ER stress-induced apoptosis. Wild-type, Bim KO, Puma KO, or Bim/Puma DKO MEFs were treated with tunicamycin or thapsigargin for 30 hr. (E) Deficiency of Bim and Puma prevents ER stress-induced BAK homo-oligomerization. Wild-type or Bim/Puma DKO MEFs were untreated or treated with tunicamycin for 30 hr. Protein lysates were subjected to Superdex 200 (HR10/30) gel filtration chromatography. Fractions were analyzed by anti-BAK immunoblots. (F) Deficiency of Bim and Puma prevents ER stress-induced BAX homo-oligomerization. The blots shown in (E) were stripped and reprobed with anti-BAX antibody. Data shown in (B) and (D) are mean ± s.d. from three independent experiments. Cell death was quantified by Annexin-V.
Figure 7. Schematic depicts the model of activation of BAX and BAK driven by “activator” BH3s
Comment in
- BAX and BAK caught in the act.
Yao Y, Marassi FM. Yao Y, et al. Mol Cell. 2009 Nov 13;36(3):353-4. doi: 10.1016/j.molcel.2009.10.023. Mol Cell. 2009. PMID: 19917244 Free PMC article.
Similar articles
- BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program.
Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Ren D, et al. Science. 2010 Dec 3;330(6009):1390-3. doi: 10.1126/science.1190217. Science. 2010. PMID: 21127253 Free PMC article. - An interconnected hierarchical model of cell death regulation by the BCL-2 family.
Chen HC, Kanai M, Inoue-Yamauchi A, Tu HC, Huang Y, Ren D, Kim H, Takeda S, Reyna DE, Chan PM, Ganesan YT, Liao CP, Gavathiotis E, Hsieh JJ, Cheng EH. Chen HC, et al. Nat Cell Biol. 2015 Oct;17(10):1270-81. doi: 10.1038/ncb3236. Epub 2015 Sep 7. Nat Cell Biol. 2015. PMID: 26344567 Free PMC article. - Bax/Bak activation in the absence of Bid, Bim, Puma, and p53.
Zhang J, Huang K, O'Neill KL, Pang X, Luo X. Zhang J, et al. Cell Death Dis. 2016 Jun 16;7(6):e2266. doi: 10.1038/cddis.2016.167. Cell Death Dis. 2016. PMID: 27310874 Free PMC article. - BCL-2 proteins and apoptosis: Recent insights and unknowns.
Edlich F. Edlich F. Biochem Biophys Res Commun. 2018 May 27;500(1):26-34. doi: 10.1016/j.bbrc.2017.06.190. Epub 2017 Jul 1. Biochem Biophys Res Commun. 2018. PMID: 28676391 Review. - Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane.
Wolf P, Schoeniger A, Edlich F. Wolf P, et al. Biochim Biophys Acta Mol Cell Res. 2022 Oct;1869(10):119317. doi: 10.1016/j.bbamcr.2022.119317. Epub 2022 Jun 22. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35752202 Review.
Cited by
- A BAK subdomain that binds mitochondrial lipids selectively and releases cytochrome C.
Dai H, Peterson KL, Flatten KS, Meng XW, Venkatachalam A, Correia C, Ramirez-Alvarado M, Pang YP, Kaufmann SH. Dai H, et al. Cell Death Differ. 2023 Mar;30(3):794-808. doi: 10.1038/s41418-022-01083-z. Epub 2022 Nov 14. Cell Death Differ. 2023. PMID: 36376382 Free PMC article. - Gender differences control the susceptibility to ER stress-induced acute kidney injury.
Hodeify R, Megyesi J, Tarcsafalvi A, Mustafa HI, Hti Lar Seng NS, Price PM. Hodeify R, et al. Am J Physiol Renal Physiol. 2013 Apr 1;304(7):F875-82. doi: 10.1152/ajprenal.00590.2012. Epub 2013 Jan 30. Am J Physiol Renal Physiol. 2013. PMID: 23364800 Free PMC article. - Regulation of Cell Death by Mitochondrial Transport Systems of Calcium and Bcl-2 Proteins.
Naumova N, Šachl R. Naumova N, et al. Membranes (Basel). 2020 Oct 21;10(10):299. doi: 10.3390/membranes10100299. Membranes (Basel). 2020. PMID: 33096926 Free PMC article. Review. - A B-Cell Superantigen Induces the Apoptosis of Murine and Human Malignant B Cells.
Lorenzo D, Duarte A, Mundiñano J, Berguer P, Nepomnaschy I, Piazzon I. Lorenzo D, et al. PLoS One. 2016 Sep 7;11(9):e0162456. doi: 10.1371/journal.pone.0162456. eCollection 2016. PLoS One. 2016. PMID: 27603942 Free PMC article. - Mitochondrial residence of the apoptosis inducer BAX is more important than BAX oligomerization in promoting membrane permeabilization.
Kuwana T, King LE, Cosentino K, Suess J, Garcia-Saez AJ, Gilmore AP, Newmeyer DD. Kuwana T, et al. J Biol Chem. 2020 Feb 7;295(6):1623-1636. doi: 10.1074/jbc.RA119.011635. Epub 2020 Jan 3. J Biol Chem. 2020. PMID: 31901077 Free PMC article.
References
- Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, Meflah K, Vallette FM, Juin P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. - PubMed
- Cartron PF, Priault M, Oliver L, Meflah K, Manon S, Vallette FM. The N-terminal end of Bax contains a mitochondrial-targeting signal. J Biol Chem. 2003;278:11633–11641. - PubMed
- Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Mitochondria primed by death signals determine cellular addiction to antiapoptotic BCL-2 family members. Cancer Cell. 2006;9:351–365. - PubMed
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. - PubMed
- Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8:705–711. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM083159/GM/NIGMS NIH HHS/United States
- R01CA125562/CA/NCI NIH HHS/United States
- R01 CA125562/CA/NCI NIH HHS/United States
- P30CA21765/CA/NCI NIH HHS/United States
- P30 CA021765/CA/NCI NIH HHS/United States
- K01CA98320/CA/NCI NIH HHS/United States
- K01 CA098320/CA/NCI NIH HHS/United States
- R01GM083159/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials