Two mechanistically distinct immune evasion proteins of cowpox virus combine to avoid antiviral CD8 T cells - PubMed (original) (raw)
Two mechanistically distinct immune evasion proteins of cowpox virus combine to avoid antiviral CD8 T cells
Minji Byun et al. Cell Host Microbe. 2009.
Abstract
Downregulation of MHC class I on the cell surface is an immune evasion mechanism shared by many DNA viruses, including cowpox virus. Previously, a cowpox virus protein, CPXV203, was shown to downregulate MHC class I. Here we report that CPXV12 is the only other MHC class I-regulating protein of cowpox virus and that it uses a mechanism distinct from that of CPXV203. Whereas CPXV203 retains fully assembled MHC class I by exploiting the KDEL-mediated endoplasmic reticulum retention pathway, CPXV12 binds to the peptide-loading complex and inhibits peptide loading on MHC class I molecules. Viruses deleted of both CPXV12 and CPXV203 demonstrated attenuated virulence in a CD8 T cell-dependent manner. These data demonstrate that CPXV12 and CPXV203 proteins combine to ablate MHC class I expression and abrogate antiviral CD8 T cell responses.
Figures
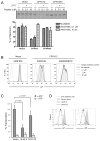
Figure 1. Identification of CPXV12 by loss-of-function screening
(A) Schematic diagram of CPXV mutants. Numbers indicate positions in kilobase (kb) corresponding to the wild-type CPXV strain Brighton Red genome. CPXV203 was replaced with GFP in CPXVΔ203 and A518Δ203. (B) MC57G cells infected with indicated CPXV mutants were analyzed for surface Kb or Db expression at 24 h post infection. (C) MC57G cells infected with CPXV mutants indicated above each histogram were harvested at 24 h post infection and their surface Kb expression levels were compared to those of CPXVΔ203- or A518Δ203-infected cells. (D) MC57G cells (Kb, Db) and L929 cells (Dk) were infected with CPXVΔ203 or CPXVΔ12Δ203 and analyzed for MHC class I expression at 24 h post infection.
Figure 2. CPXV12 delays MHC class I trafficking
(A) Surface expression levels of Kb and Db were measured in C1498 cells transduced with either vector alone or CPXV12. GFP serves as a marker for transduction. (B) Intracellular trafficking of Kb molecules was examined in the presence and absence of CPXV12. C1498 cells transduced with either vector alone or CPXV12 were sorted several times until the GFP positive population was >95% (data not shown). They were labeled with [35S]-Met/Cys for 10 min and chased for the indicated periods of time. Kb molecules were immunoprecipitated from cell lysates and treated with Endo H prior to SDS-PAGE. The proportion of Endo H-resistant form at each chase time point was plotted. A representative result of three independent experiments is shown. EH-R, Endo H-resistant; EH-S, Endo H-sensitive. (C) C1498 cells transduced with either vector alone or CPXV12 were sorted several times until the GFP positive population was >95% (data not shown). MC57G cells were infected with either CPXVΔ12Δ203 or CPXVΔ203 at a MOI of 3. Cell lysates were treated with Endo H where indicated, and Kb heavy chain was detected by immunoblotting.
Figure 3. CPXV12 inhibits MHC class I dissociation from the PLC
(A) Digitonin lysates of C1498 cells transduced with either vector alone or CPXV12 (left four lanes) and of MC57G cells infected with either CPXVΔ12Δ203 or CPXVΔ203 (right two lanes) were subjected to immunoprecipitation with anti-HA or anti-TAP1 serum. Precipitated proteins were separated by SDS-PAGE and blotted for indicated proteins. N.D., not determined. (B) C1498 cells transduced with either vector alone or CPXV12 were labeled with [35S]-Met/Cys for 10 min, and then chased for the indicated periods of time. Cells were lysed with 1% digitonin, and subjected to immunoprecipitation with anti-TAP1 serum. From the co-precipitated proteins, Kb was further purified by re-immunoprecipitation with anti-Kb serum. Protein bands corresponding to Kb heavy chain (HC) and β2m are indicated. Asterisk (*) indicates non-specific protein. The levels of Kb heavy chain at each time-point compared to the initial amounts (set as 100%) are plotted in the bottom graph. Mean ± standard error of mean (SEM) from two independent experiments is shown.
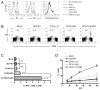
Figure 4. CPXV12 impairs peptide loading
(A) C1498 cells transduced with indicated constructs were labeled with [35S]-Met/Cys for 10 min and chased for 60 min. Cell lysates were incubated with indicated concentrations of peptide VNVDYSKL at 4°C for 30 min, and subsequently incubated at 37°C for 30 min. Kb was immunoprecipitated with monoclonal antibody B8-24-3, which recognizes peptide-bound Kb. Band intensities were quantified and plotted as percent of maximum, which represents the amount of Kb before the 37°C-incubation (set as 100%). Mean ± SEM from three independent experiments is shown. (B) C1498 cells transduced with vector alone or CPXV12 were incubated with indicated concentrations of SIINFEKL or ASNENMETM peptide in the culture medium for 16 h at 37°C, and analyzed for surface Kb expression by flow cytometry. (C) Peptide transport activity of TAP was assessed in C1498 cells transduced with vector alone, BHV-1 UL49.5, or CPXV12. Translocation of the fluorescently-labeled peptide CVNKTERAY was evaluated in the presence or absence of ATP. Peptide transport is expressed as percentage of translocation, relative to the translocation observed in control cells (set as 100%). Statistical significance was determined by Student’s t-test. (D) Surface levels of Kb and Db were compared between C1498 cells transduced with indicated constructs.
Figure 5. Evasion of antiviral CD8 T cell responses by CPXV12 and CPXV203
C57BL/6 mice were intranasally infected with 1×104 PFU of wtCPXV. Splenocytes were harvested at day 7 post infection. MC57G cells were mock infected or infected with indicated viruses at a MOI of 2 for 18 h. (A) Surface expression levels of Kb, Db, and poxvirus HA on MC57G cells were analyzed by flow cytometry. (B) Splenocytes were incubated with MC57G cells for 7 h, and analyzed for IFN-γ by intracellular staining followed by flow cytometry. Numbers indicate percentage of IFN-γ+ cells among total CD8+ lymphocytes. A representative result of three similar experiments is shown. (C) Same as (B) except mean ± SEM from three independent experiments is shown. Statistical significance was determined by Student’s t-test. *, p < 0.05; ns, not significant. (D) Splenocytes were co-incubated with 51Cr-labeled MC57G cells for 4 h. Lysis of MC57G cells was measured by 51Cr-release assay. E/T represents effector (splenocytes) to target (MC57G cells) ratio. Mean ± SEM from three independent experiments is shown.
Figure 6. CPXV12 and CPXV203 abrogate CD8 T cell immunity against CPXV in vivo
(A) 9-week-old C57BL/6 mice were infected intranasally with 5×104 PFU of the indicated viruses and monitored for survival. Results were pooled from two independent experiments. (B) One-step growth curves for the indicated viruses were obtained in Vero cells. Genome copy numbers were determined by quantitative PCR. Mean ± SEM of triplicates is shown. (C) 9-week-old C57BL/6 mice were infected intranasally with indicated viruses. Spleens from infected mice were harvested at day 7 or day 10 post infection, and the viral loads were determined by quantitative PCR. Dotted lines indicate limits of detection. Statistical significance was determined by Student’s t-test. (D) 9-week-old C57BL/6 mice were infected intranasally with 1×104 PFU of the indicated viruses and monitored for survival. Starting two days prior to infection, mice were treated with 1 mg of either isotype-control or CD8-depleting antibody every fourth day throughout the monitoring period. Results were pooled from two independent experiments.
Comment in
- Jenner's irony: cowpox taps into T cell evasion.
Wilkinson GW, Lehner PJ. Wilkinson GW, et al. Cell Host Microbe. 2009 Nov 19;6(5):395-7. doi: 10.1016/j.chom.2009.11.001. Cell Host Microbe. 2009. PMID: 19917492
Similar articles
- Structural mechanism of ER retrieval of MHC class I by cowpox.
McCoy WH 4th, Wang X, Yokoyama WM, Hansen TH, Fremont DH. McCoy WH 4th, et al. PLoS Biol. 2012;10(11):e1001432. doi: 10.1371/journal.pbio.1001432. Epub 2012 Nov 27. PLoS Biol. 2012. PMID: 23209377 Free PMC article. - Cowpox virus inhibits the transporter associated with antigen processing to evade T cell recognition.
Alzhanova D, Edwards DM, Hammarlund E, Scholz IG, Horst D, Wagner MJ, Upton C, Wiertz EJ, Slifka MK, Früh K. Alzhanova D, et al. Cell Host Microbe. 2009 Nov 19;6(5):433-45. doi: 10.1016/j.chom.2009.09.013. Cell Host Microbe. 2009. PMID: 19917498 Free PMC article. - Cowpox virus exploits the endoplasmic reticulum retention pathway to inhibit MHC class I transport to the cell surface.
Byun M, Wang X, Pak M, Hansen TH, Yokoyama WM. Byun M, et al. Cell Host Microbe. 2007 Nov 15;2(5):306-15. doi: 10.1016/j.chom.2007.09.002. Cell Host Microbe. 2007. PMID: 18005752 - Cowpox virus employs a two-pronged strategy to outflank MHCI antigen presentation.
McCoy WH 4th, Wang X, Yokoyama WM, Hansen TH, Fremont DH. McCoy WH 4th, et al. Mol Immunol. 2013 Sep;55(2):156-8. doi: 10.1016/j.molimm.2012.11.011. Epub 2013 Jan 10. Mol Immunol. 2013. PMID: 23312338 Free PMC article. Review. - Recent advances in viral evasion of the MHC Class I processing pathway.
Schuren AB, Costa AI, Wiertz EJ. Schuren AB, et al. Curr Opin Immunol. 2016 Jun;40:43-50. doi: 10.1016/j.coi.2016.02.007. Epub 2016 Apr 8. Curr Opin Immunol. 2016. PMID: 27065088 Review.
Cited by
- Structural mechanism of ER retrieval of MHC class I by cowpox.
McCoy WH 4th, Wang X, Yokoyama WM, Hansen TH, Fremont DH. McCoy WH 4th, et al. PLoS Biol. 2012;10(11):e1001432. doi: 10.1371/journal.pbio.1001432. Epub 2012 Nov 27. PLoS Biol. 2012. PMID: 23209377 Free PMC article. - Interferon-γ mediates chemokine-dependent recruitment of natural killer cells during viral infection.
Pak-Wittel MA, Yang L, Sojka DK, Rivenbark JG, Yokoyama WM. Pak-Wittel MA, et al. Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):E50-9. doi: 10.1073/pnas.1220456110. Epub 2012 Dec 17. Proc Natl Acad Sci U S A. 2013. PMID: 23248310 Free PMC article. - Direct Priming of CD8+ T Cells Persists in the Face of Cowpox Virus Inhibitors of Antigen Presentation.
Lin LCW, Croft SN, Croft NP, Wong YC, Smith SA, Tang SS, Purcell AW, Tscharke DC. Lin LCW, et al. J Virol. 2021 Apr 26;95(10):e00186-21. doi: 10.1128/JVI.00186-21. Epub 2021 Mar 10. J Virol. 2021. PMID: 33692206 Free PMC article. - Cowpox virus induces interleukin-10 both in vitro and in vivo.
Spesock AH, Barefoot BE, Ray CA, Kenan DJ, Gunn MD, Ramsburg EA, Pickup DJ. Spesock AH, et al. Virology. 2011 Aug 15;417(1):87-97. doi: 10.1016/j.virol.2011.05.010. Epub 2011 Jun 11. Virology. 2011. PMID: 21658738 Free PMC article. - Crystal structure of the cowpox virus-encoded NKG2D ligand OMCP.
Lazear E, Peterson LW, Nelson CA, Fremont DH. Lazear E, et al. J Virol. 2013 Jan;87(2):840-50. doi: 10.1128/JVI.01948-12. Epub 2012 Oct 31. J Virol. 2013. PMID: 23115291 Free PMC article.
References
- Ahn K, Gruhler A, Galocha B, Jones TR, Wiertz EJ, Ploegh HL, Peterson PA, Yang Y, Fruh K. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity. 1997;6:613–621. - PubMed
- Andersson M, Paabo S, Nilsson T, Peterson PA. Impaired intracellular transport of class I MHC antigens as a possible means for adenoviruses to evade immune surveillance. Cell. 1985;43:215–222. - PubMed
- Boname JM, de Lima BD, Lehner PJ, Stevenson PG. Viral degradation of the MHC class I peptide loading complex. Immunity. 2004;20:305–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 AI057160-010001/AI/NIAID NIH HHS/United States
- U54 AI057160-05S10014/AI/NIAID NIH HHS/United States
- R01 AI019687/AI/NIAID NIH HHS/United States
- U54 AI057160/AI/NIAID NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- U54 AI057160-065904/AI/NIAID NIH HHS/United States
- U54-AI057160/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials