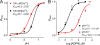Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity - PubMed (original) (raw)
Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity
Jianyang Du et al. J Gen Physiol. 2009 Dec.
Abstract
TRPM2 is a Ca(2+)-permeable nonselective cation channel that plays important roles in oxidative stress-mediated cell death and inflammation processes. However, how TRPM2 is regulated under physiological and pathological conditions is not fully understood. Here, we report that both intracellular and extracellular protons block TRPM2 by inhibiting channel gating. We demonstrate that external protons block TRPM2 with an IC(50) of pH(o) = 5.3, whereas internal protons inhibit TRPM2 with an IC(50) of pH(i) = 6.7. Extracellular protons inhibit TRPM2 by decreasing single-channel conductance. We identify three titratable residues, H958, D964, and E994, at the outer vestibule of the channel pore that are responsible for pH(o) sensitivity. Mutations of these residues reduce single-channel conductance, decrease external Ca(2+) ([Ca(2+)](o)) affinity, and inhibit [Ca(2+)](o)-mediated TRPM2 gating. These results support the following model: titration of H958, D964, and E994 by external protons inhibits TRPM2 gating by causing conformation change of the channel, and/or by decreasing local Ca(2+) concentration at the outer vestibule, therefore reducing [Ca(2+)](o) permeation and inhibiting [Ca(2+)](o)-mediated TRPM2 gating. We find that intracellular protons inhibit TRPM2 by inducing channel closure without changing channel conductance. We identify that D933 located at the C terminus of the S4-S5 linker is responsible for intracellular pH sensitivity. Replacement of Asp(933) by Asn(933) changes the IC(50) from pH(i) = 6.7 to pH(i) = 5.5. Moreover, substitution of Asp(933) with various residues produces marked changes in proton sensitivity, intracellular ADP ribose/Ca(2+) sensitivity, and gating profiles of TRPM2. These results indicate that D933 is not only essential for intracellular pH sensitivity, but it is also crucial for TRPM2 channel gating. Collectively, our findings provide a novel mechanism for TRPM2 modulation as well as molecular determinants for pH regulation of TRPM2. Inhibition of TRPM2 by acidic pH may represent an endogenous mechanism governing TRPM2 gating and its physiological/pathological functions.
Figures
Figure 1.
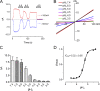
Inhibitory effects of acidic pHo on TRPM2. (A) Both inward and outward currents of TRPM2 elicited by voltage ramps ranging from −100 to +100 mV were completely and reversibly inhibited by pHo 4.5. Note that pHo 4.0 irreversibly blocked TRPM2. Internal pipette solutions contained 100 nM [Ca2+]i buffered by 1 mM EGTA and 200 µM ADPR. Inward and outward currents were measured at −100 and +100 mV, respectively. (B) Representative recordings of TRPM2 by ramp protocols ranging from −100 to +100 mV at the indicated pHo. (C) Mean current amplitude of TRPM2 at the indicated pHo (mean ± SEM; n = 9). (D) Dose–response curve of pHo constructed by normalizing current amplitude at the indicated pHo to the maximal current amplitude at pH7.4. Best fit of the normalized data yielded an IC50 of 5.3 ± 0.05 pH units (mean ± SEM; n = 9; Hill coefficient, nH = 1.3).
Figure 2.
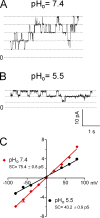
Effects of external protons on single-channel conductance. Single-channel currents were recorded under inside-out configuration with external solutions (pipette solutions) containing 2 mM Ca2+ and internal solutions (bath solutions) containing nominal Ca2+-free Tyrode's solution and 200 µM ADPR (pHo 7.2). (A) Representative recording of TRPM2 at +80 mV with the external solution (pipette solution) at pHo 7.4. (B) Single-channel recordings of TRPM2 at +80 mV with the external solution (pipette solution) at pHo 5.5. (C) Average current amplitude at each voltage plotted against the test potential. Linear regression yielded single-channel conductances of 75.4 ± 0.8 pS (mean ± SEM; n = 7∼9) at pHo 7.4 and 43.2 ± 0.9 pS (mean ± SEM; n = 7∼9) at pHo 5.5.
Figure 3.
Effects of external and internal Ca2+ on protons' inhibition of TRPM2. (A) Concentration-dependent effects of external acidic pH on TRPM2 in the solutions containing 200 µM and 2 mM [Ca2+]o, respectively. Whole cell currents were recorded with pipette solutions containing minimal [Ca2+]i buffered by 10 mM EGTA and 500 µM ADPR. Ramp protocol (−100 to +100 mV) was used to elicit TRPM2 currents. Current amplitude at +100 mV was measured and normalized to the maximal current amplitude at pH 7.4. The proton dose–response curve was rightward shifted at 200 µM of external [Ca2+]o, and the IC50 was changed from 5.4 ± 0.05 pH units (2 mM [Ca2+]o; n = 8) to 7.5 ± 0.1 pH units (200 µM [Ca2+]o; n = 8). (B) Effects of internal [Ca2+]i on concentration-dependent inhibition of TRPM2 by external protons. Whole cell currents of TRPM2 were recorded in the 2 mM of external Ca2+ with pipette solutions containing 100 nM or 100 µM [Ca2+]i in the presence of 200 µM [ADPR]i. Protons produced similar inhibitory effects on TRPM2 at 100 nM (n = 9) and 100 µM (n = 6) of internal [Ca2+]i (with 200 µM ADPR), respectively.
Figure 4.
TRPM2 is not permeable to protons. (A) Inward currents of TRPM2 recorded by holding the TRPM2-transfected cell at −100 mV. No current was activated in NMDH-Cl solutions at pHo 4.5 or 7.4, where Tyrode's solution generated large inward current. (B) In mock-transfected cells, NMDH-Cl solutions at pHo 4.5 or 7.4 could not induce any current. (C) TRPM2 current elicited by the ramp protocol (−100 to +100 mV) in Tyrode's solution (red). NMDG-Glu at pHo 4.5 did not induce any inward proton current (green). The pipette solution for this experiment contained NMDG-Glu to favor proton permeation. (D) Inward current of TRPM2 recorded at various external solutions obtained in C. Currents were measured at −100 mV and plotted as a function of time. (E) Effects of holding potentials on the inhibition of TRPM2 by external protons. Dose–response curves were constructed by normalizing the inward currents (−100 mV) at each pHo to the maximal current amplitude (−100 mV) obtained at pHo 7.4. The IC50s were 5.3 ± 0.05 (n = 9) at HP = 0 mV and 5.5 ± 0.14 (n = 9; P > 0.05) at HP = −100 mV. (F) Inhibition of TRPM2 by acidic pHo (pHo = 4.0) could not be reversed by high pHi. Inward currents (−100 mV) and outward currents (+100 mV) plotted against time under the indicated conditions. TRPM2 currents were elicited by voltage ramps with the CsSO3CH3 pipette solution containing 100 nM Ca2+ and 200 µM ADPR. Note that TRPM2 was completely and irreversibly blocked by external solutions at pHo 4.0. 30 mM NH4Cl, used to increase intracellular pH, could not reverse the effects of pHo 4. Similar results were obtained in five separate experiments.
Figure 5.
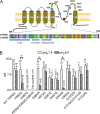
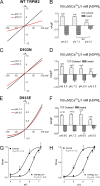
Molecular basis for TRPM2 sensitivity to acidic pHo. (A) Schematic structure of TRPM2 and positions of substituted amino acid residues in the putative pore region of hTRPM2 (top). Alignment of the residues in the putative pore region of mTRPM2 and hTRPM2 (bottom). (B) Average current amplitude of WT TRPM2 and the mutants at pHo 7.4 and 6.0. TRPM2 currents were elicited by voltage ramps, and the outward currents at +100 mV were measured. E960Q and the triple mutant H958Q-E960Q-D964N were nonfunctional channels. *, P < 0.05.
Figure 6.
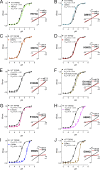
Changes in the pHo sensitivity of TRPM2 mutants. (A–G) Dose–response curves of the effects of acidic pHo on mutants H973Q, Q981E, D987Q, H995Q, D1002N, E1010Q/D1012N, and E1022Q. Current amplitudes at the indicated pHs were normalized to the maximal current amplitude at pHo 7.4. Inset panels illustrate original recordings of WT TRPM2 and the mutants at pH 7.4 and 6.0. Best fit of the dose–response curves yielded IC50s for the mutants indistinguishable from that of WT TRPM2. (H–J) Altered pHo sensitivity in H958Q, D964N, and E994Q compared with WT TRPM2. Dose–response curves were rightward shifted, and the IC50s were changed by ∼1 pH unit for H958Q, D964N, and E994Q, respectively. Inset panels illustrate representative recordings at pH 7.4 and 6.0. Note that the linear I-V relation of WT TRPM2 was not changed in H958Q, D964N, and E994Q. The Hill coefficient was nH = 1.4 ± 0.1 for WT TRPM2, nH = 1.0 ± 0.1 for H958Q, nH = 1.7 ± 0.3 for D964N, and nH = 3.2 ± 0.3 for E994Q.
Figure 7.
Changes in [Ca2+]o sensitivity of TRPM2 mutants H958Q, D964N, and E994Q. (A–D) Inward and outward currents of WT TRPM2 (A), H958Q (B), D964N (C), and E994Q (D) activated by the indicated [Ca2+]o. (E) Mean current amplitude of WT TRPM2, H958Q, D964N, and E994Q at the indicated [Ca2+]o. (F) Concentration-dependent effects of [Ca2+]o. Currents were normalized to the maximal value obtained at 2 mM [Ca2+]o. EC50s for Ca2+-mediated activation were 131 ± 10.8 µM (nH = 3.3 ± 0.5; n = 8) for WT TRPM2, 239 ± 25 µM (nH = 2.7 ± 0.5; n = 8) for H958Q, 256 ± 30.2 µM (nH = 2.8 ± 0.4; n = 8) for D964N, and 298 ± 52.6 µM (nH = 2.6 ± 0.4; n = 8) for E994Q.
Figure 8.
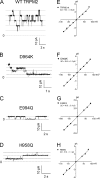
Single-channel conductance of D964K, E994Q, and H958Q. (A–D) Representative recordings of single-channel openings of WT TRPM2, D964K, E994Q, and H958Q at +80 mV in inside-out patches. The external (pipette) solution was normal Tyrode's solution; the internal (bath) solution was nominal Ca2+-free solution with 200 µM ADPR. (E–H) Single-channel conductances obtained by linear regression of single-channel current amplitude at the indicated voltages. The conductance is 59.9 ± 1.0 pS for D964K (n = 8), 49.5 ± 0.4 pS (n = 4) for E994Q, and 57.8 ± 1.1 pS (n = 4) for H958Q.
Figure 9.
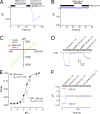
Effects of acidic pHi on TRPM2 whole cell currents. (A) TRPM2 currents recorded at pHi 7.2, 6.5, and 5.5. Note that there was a delay for activation of TRPM2 at pHi 6.5, and a much longer delay for TRPM2 activation at pHi 5.5. (B) Typical I-V relationship of TRPM2 whole cell current at different pHis. (C) The inhibitory effect of pHi 5.5 on TRPM2 was reversed by perfusing the cells with extracellular solutions containing 30 mM NH4Cl. (D) Representative recordings of TRPM2 in the presence of NH4Cl. NH4Cl increased both inward and outward currents of TRPM2.
Figure 10.
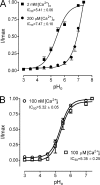
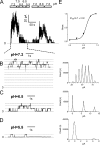
TRPM2 is an intracellular acidic pH sensor. (A) Effects of low pHi on single-channel currents of TRPM2 obtained from inside-out patches. Single-channel currents were recorded at +80 mV, with the pipette solution containing 2 mM [Ca2+]o (external solution) and the bath solution (internal solution) containing 100 nM [Ca2+]i and 200 µM [ADPR]i. Intracellular low pH blocked TRPM2 channel openings (+80 mV), and the effect was partially reversed by pHi 8.0. (B–D) Single-channel currents recorded at the indicated pHis. Current amplitude histograms illustrate the number of open channels (right). (B) At pHi 7.2, there were >10 open channels. (C) At pHi 6.5, the number of open channels decreased to two. Average current amplitude (6.13 ± 0.02 pA; n = 10) estimated a single-channel conductance of 76.6 pS. (D) Channel opening was almost eliminated at pHi 5.5. Single-channel conductance estimated from the mean current amplitude (6.06 ± 0.02 pA; n = 10) was 75.7 pS. (E) Concentration-dependent effects of internal protons on TRPM2. Macroscopic currents of TRPM2 were obtained in inside-out patches. A best fit of the normalized current amplitude yielded an IC50 of 6.7 ± 0.02 pH units (nH = 3.0 ± 0.3; n = 10 for each indicated pH). Analysis using currents recorded at +80 mV or at −80 mV produced identical IC50s.
Figure 11.
Effects of internal protons on [Ca2+]i- and [ADPR]i-induced TRPM2 gating. (A) Concentration-dependent effects of internal protons on TRPM2 at various concentrations of internal [Ca2+]i. TRPM2 currents were elicited by voltage ramps (−100 to +100 mV; HP = 0 mV) under inside-out configuration, and the macroscopic current amplitude was measured at +100 mV. The intracellular solution (bath solution) contained 200 µM ADPR with 100 µM or 100 nM Ca2+; the external solution (pipette solution) was normal Tyrode's solution containing 2 mM Ca2+. Best fit of normalized current yielded an IC50 of 6.7 ± 0.02 (n = 10) at 100 nM [Ca2+]i and 6.3 ± 0.02 (n = 11; P < 0.01) at 100 uM [Ca2+]i. (B) Effects of internal low pHi on [ADPR]i-induced TRPM2 gating. Macroscopic currents were recorded using inside-out patches by voltage ramps (−100 to +100 mV; HP = 0 mV) in the nominal Ca2+-free (∼10 µM) internal solutions at pHi 7.2 or 6.7 with various concentrations of ADPR. The EC50s of ADPR were 2.7 ± 0.9 µM (n = 5) at pHi 7.2 and 154.2 ± 27 µM at pHi 6.7 (n = 5).
Figure 12.
An intracellular residue, D933, influences TRPM2 channel gating. (A) Current amplitude measured at +100 and −100 mV of D933E, D933N, D933H, D933K, and D933A compared with WT TRPM2. The mutant channels were activated by intracellular solutions containing 100 µM Ca2+ and 1 mM ADPR, whereas WT TRPM2 was activated by 100 nM Ca2+/200 µM ADPR. Note that D933E and D933N quickly inactivated after activation. (B) Mean current amplitude of WT TRPM2 and the mutants recorded with 100 nM Ca2+/200 µM ADPR. (C) Mean current amplitude of the mutants activated by 100 µM Ca2+/1 mM ADPR. (D–I) I-V relationship of D933N, D933E, D933H, D933K, and D933A compared with that of WT TRPM2. Both D933E and D933H displayed smaller inward currents than that of D933N.
Figure 13.
D933 is an intracellular pH sensor. (A and B) WT TRPM2 activated by 100 µM [Ca2+]i/1 mM [ADPR]i was largely blocked at pHi 6.0. (C and D) Effects of acidic pHi on D933N activated by 100 µM [Ca2+]i/1 mM [ADPR]i. Acidic pHi 6.0 did not change current amplitude, whereas pHi 5.0 significantly inhibited D933N currents. (E and F) Effects of pHi on D933E. A significant decrease in current amplitude was observed at pH 5.0, but not pH 6.0. (G and H) Concentration-dependent effects of acidic pHi on D933N and D933E. IC50s obtained by best fit of the dose–response curves were 5.5 ± 0.4 pH units for D933N and 5.3 ± 0.2 pH units for D933E. **, P < 0.01.
Similar articles
- Four Ca2+ ions activate TRPM2 channels by binding in deep crevices near the pore but intracellularly of the gate.
Csanády L, Törocsik B. Csanády L, et al. J Gen Physiol. 2009 Feb;133(2):189-203. doi: 10.1085/jgp.200810109. J Gen Physiol. 2009. PMID: 19171771 Free PMC article. - The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification.
Starkus JG, Fleig A, Penner R. Starkus JG, et al. J Physiol. 2010 Apr 15;588(Pt 8):1227-40. doi: 10.1113/jphysiol.2010.187476. Epub 2010 Mar 1. J Physiol. 2010. PMID: 20194125 Free PMC article. - State-dependent inhibition of TRPM2 channel by acidic pH.
Yang W, Zou J, Xia R, Vaal ML, Seymour VA, Luo J, Beech DJ, Jiang LH. Yang W, et al. J Biol Chem. 2010 Oct 1;285(40):30411-8. doi: 10.1074/jbc.M110.139774. Epub 2010 Jul 26. J Biol Chem. 2010. PMID: 20660597 Free PMC article. - TRPM2.
Faouzi M, Penner R. Faouzi M, et al. Handb Exp Pharmacol. 2014;222:403-26. doi: 10.1007/978-3-642-54215-2_16. Handb Exp Pharmacol. 2014. PMID: 24756715 Review. - TRPM2: a multifunctional ion channel for calcium signalling.
Sumoza-Toledo A, Penner R. Sumoza-Toledo A, et al. J Physiol. 2011 Apr 1;589(Pt 7):1515-25. doi: 10.1113/jphysiol.2010.201855. Epub 2010 Dec 6. J Physiol. 2011. PMID: 21135052 Free PMC article. Review.
Cited by
- Two sets of amino acids of the domain I of Cav2.3 Ca(2+) channels contribute to their high sensitivity to extracellular protons.
Cens T, Rousset M, Charnet P. Cens T, et al. Pflugers Arch. 2011 Aug;462(2):303-14. doi: 10.1007/s00424-011-0974-x. Epub 2011 May 25. Pflugers Arch. 2011. PMID: 21611731 - Functions of TRPs in retinal tissue in physiological and pathological conditions.
do Nascimento THO, Pereira-Figueiredo D, Veroneze L, Nascimento AA, De Logu F, Nassini R, Campello-Costa P, Faria-Melibeu ADC, Souza Monteiro de Araújo D, Calaza KC. do Nascimento THO, et al. Front Mol Neurosci. 2024 Sep 25;17:1459083. doi: 10.3389/fnmol.2024.1459083. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39386050 Free PMC article. Review. - Bilirubin gates the TRPM2 channel as a direct agonist to exacerbate ischemic brain damage.
Liu HW, Gong LN, Lai K, Yu XF, Liu ZQ, Li MX, Yin XL, Liang M, Shi HS, Jiang LH, Yang W, Shi HB, Wang LY, Yin SK. Liu HW, et al. Neuron. 2023 May 17;111(10):1609-1625.e6. doi: 10.1016/j.neuron.2023.02.022. Epub 2023 Mar 14. Neuron. 2023. PMID: 36921602 Free PMC article. - Histidine residues in the Na+-coupled ascorbic acid transporter-2 (SVCT2) are central regulators of SVCT2 function, modulating pH sensitivity, transporter kinetics, Na+ cooperativity, conformational stability, and subcellular localization.
Ormazabal V, Zuñiga FA, Escobar E, Aylwin C, Salas-Burgos A, Godoy A, Reyes AM, Vera JC, Rivas CI. Ormazabal V, et al. J Biol Chem. 2010 Nov 19;285(47):36471-85. doi: 10.1074/jbc.M110.155630. Epub 2010 Sep 14. J Biol Chem. 2010. PMID: 20843809 Free PMC article. - Role of the Intracellular Sodium Homeostasis in Chemotaxis of Activated Murine Neutrophils.
Najder K, Rugi M, Lebel M, Schröder J, Oster L, Schimmelpfennig S, Sargin S, Pethő Z, Bulk E, Schwab A. Najder K, et al. Front Immunol. 2020 Sep 8;11:2124. doi: 10.3389/fimmu.2020.02124. eCollection 2020. Front Immunol. 2020. PMID: 33013896 Free PMC article.
References
- Carter R.N., Tolhurst G., Walmsley G., Vizuete-Forster M., Miller N., Mahaut-Smith M.P. 2006. Molecular and electrophysiological characterization of transient receptor potential ion channels in the primary murine megakaryocyte. J. Physiol. 576:151–162 10.1113/jphysiol.2006.113886 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous