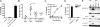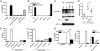Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome - PubMed (original) (raw)
Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome
Shankar S Iyer et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Dying cells are capable of activating the innate immune system and inducing a sterile inflammatory response. Here, we show that necrotic cells are sensed by the Nlrp3 inflammasome resulting in the subsequent release of the proinflammatory cytokine IL-1beta. Necrotic cells produced by pressure disruption, hypoxic injury, or complement-mediated damage were capable of activating the Nlrp3 inflammasome. Nlrp3 inflammasome activation was triggered in part through ATP produced by mitochondria released from damaged cells. Neutrophilic influx into the peritoneum in response to necrotic cells in vivo was also markedly diminished in the absence of Nlrp3. Nlrp3-deficiency moreover protected animals against mortality, renal dysfunction, and neutrophil influx in an in vivo renal ischemic acute tubular necrosis model. These findings suggest that the inhibition of Nlrp3 inflammasome activity can diminish the acute inflammation and damage associated with tissue injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
Inflammation induced in vivo by pressure-disrupted necrotic cells is dependent on the Nlrp3 inflammasome. (A–C) Neutrophil influx into the peritoneum of WT, IL-1R-, caspase-1-, ASC-, and Nlrp3-deficient mice 16 h after i.p. challenge with pressure-disrupted B16 cells (B16). Control WT mice were challenged i.p. with PBS. *, P = 0.0017; **, P = 0.0021; ***, P = 0.0159; ****, P = 0.0240.
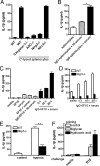
Fig. 2.
Cellular injury induced by pressure-disruption is sensed by the Nlrp3 inflammasome. (A) LPS-primed WT Mϕ were stimulated with either UV-treated, freeze-thawed, or pressure-disrupted B16 cells at a ratio of 10 necrotic cells per Mϕ; culture supernatants were collected 12 h later, and IL-1β release was measured by ELISA. (B) Neutrophil influx into the peritoneum of WT and caspase-1-deficient mice 16 h after i.p. challenge with either 1 × 107 pressure-disrupted B16 cells (B16) or freeze-thawed B16 cells. *, P = 0.0041. (C and D) LPS-primed WT, Nlrp3-, caspase-1-, or ASC-deficient Mϕ were stimulated with pressure-disrupted B16 cells and supernatants collected 12 h later or at the indicated time. IL-1β release was measured by ELISA. (E) Lysates from LPS-primed WT, Nlrp3-, or ASC-deficient Mϕ stimulated with pressure-disrupted B16 cell for 12 h were immunoblotted with antibodies against the p10 subunit of caspase-1, IL-1β, and GAPDH. (A, C, and D) Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of two (A) and three (C and D) separate experiments.
Fig. 3.
Complement- and hypoxia-induced cellular injury is sensed by the Nlrp3 inflammasome. (A) LPS-primed WT, caspase-1-, ASC-, Nlrp3-, or Nlrc4-deficient Mϕ were stimulated with complement-lysed splenocytes at a ratio of 25 splenocytes per Mϕ; supernatants were collected 12 h later, and IL-1β release assessed by ELISA. (B–D) Splenocytes or the hybridoma cell line 6F10 were either left untreated or opsonized with anti-MHC class II Aβ(b) IgG2a antibody followed by incubation with human serum (serum) or heat-inactivated human serum (HI-serum). LPS-primed WT or Nlrp3−/− Mϕ were stimulated with complement-damaged cells at a ratio of 25 splenocytes per Mϕ, 20 6F10 cells per Mϕ, or as indicated; supernatants were collected 12 h later, and IL-1β release assessed by ELISA. *, P = 0.0003. (E) LPS-primed WT or Nlrp3−/− Mϕ were stimulated with B16 cells grown under normoxic (control) or hypoxic conditions at a ratio of five cells per Mϕ; supernatants were collected 12 h later, and IL-1β release assessed by ELISA. **, P = 0.0013. (F) WT Mϕ were primed with either biglycan (8 μg/mL) or hyaluronic acid (25 μg/mL) for 12 h followed by stimulation with pressure-disrupted B16 cell at a ratio of 10 necrotic cells per Mϕ; supernatants were collected 12 h later, and IL-1β release measured by ELISA. (A–F) Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of two (C–F) and three (A and B) separate experiments.
Fig. 4.
Mitochondria released from necrotic cells stimulate Nlrp3 inflammasome activation. (A) LPS-primed WT Mϕ were stimulated with pressure-disrupted B16 cells or nuclei, plasma membrane, cytosol, and mitochondrial fractions derived from B16 cells at a ratio of five cell equivalents per Mϕ; supernatants were collected 12 h later, and IL-1β release was measured by ELISA. (B) LPS-primed WT, caspase-1-, ASC-, Nlrp3-, or Nlrc4-deficient Mϕ were left untreated or stimulated with mitochondria (100 μg/mL); supernatants were collected 12 h later, and IL-1β release was assessed by ELISA. (C) Lysates from LPS-primed WT, Nlrp3-, or ASC-deficient Mϕ stimulated with mitochondria for 12 h were immunoblotted with antibodies against the p10 subunit of caspase-1 and GAPDH. (D) Neutrophil influx into the peritoneum of WT and Nlrp3-deficient mice 16 h after i.p. challenge with mitochondria isolated from 1 × 107 B16 cells. Control WT mice were challenged i.p. with homogenization buffer. *, P = 0.0317. (E) Mitochondria were treated with rotenone (10 μM), myxothiazol (10 μM), apyrase (1 U) for 20 min or heat-treated at 65 °C for 20 min, washed, and ATP content quantified. (F) LPS-primed WT Mϕ were stimulated with mitochondria (100 μg/mL) that had been treated as described; supernatants were collected 12 h later, and IL-1β release was measured by ELISA. (G) LPS-primed WT or P2X7R-deficient Mϕ were stimulated with ATP (5 mM), silica (50 μg/cm2), pressure-disrupted B16 cells, or mitochondria; supernatants were collected 12 h later, and IL-1β release was measured by ELISA. **, P = 0.0041; ***, P = 0.0009. Determinations were performed in triplicate and expressed as the mean ± SEM. Results are representative of two (A, E, and F) and three (B and G) separate experiments.
Fig. 5.
Nlrp3-deficiency protects animals against mortality, renal dysfunction, and impairs an inflammatory response during renal ischemic acute tubular necrosis. (A) Relative Nlrp3 mRNA levels were measured in kidneys of WT mice 1 day after nonlethal renal I/R or sham operation. (B) Tubular necrosis score of WT mice (n = 8) and Nlrp3-deficient mice (n = 8) after nonlethal renal I/R injury using PAS-D-stained renal tissue sections. (C) Survival of Nlrp3-deficient mice (n = 14) compared to WT mice (n = 13) after lethal renal ischemia. (D and E) Renal dysfunction of Nlrp3-deficient mice (n = 8) compared to WT mice (n = 8) as reflected by increased levels of urea (D) and creatinine (E) in plasma after nonlethal renal acute tubular necrosis. (F and G) Neutrophil influx in kidneys from WT and Nlrp3-deficient mice 1 day after renal I/R or sham operation as assessed by immunohistochemistry (F) and counted in at least 10 randomly selected high-power fields in the outer medulla (G). Original magnification of pictures, ×400. (H and I) Total KC and IL-1β levels in kidneys from Nlrp3-deficient and WT mice subjected for 1 day to renal I/R injury. *, P < 0.05.
Comment in
- Release of the mitochondrial endosymbiont helps explain sterile inflammation.
Masters SL, Walsh PT. Masters SL, et al. Proc Natl Acad Sci U S A. 2010 Mar 9;107(10):E32. doi: 10.1073/pnas.1000366107. Proc Natl Acad Sci U S A. 2010. PMID: 20215475 Free PMC article. No abstract available.
Similar articles
- Histones trigger sterile inflammation by activating the NLRP3 inflammasome.
Allam R, Darisipudi MN, Tschopp J, Anders HJ. Allam R, et al. Eur J Immunol. 2013 Dec;43(12):3336-42. doi: 10.1002/eji.201243224. Epub 2013 Sep 6. Eur J Immunol. 2013. PMID: 23964013 - Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors.
Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, Young MF, Bruckner P, Pfeilschifter J, Schaefer RM, Gröne HJ, Schaefer L. Babelova A, et al. J Biol Chem. 2009 Sep 4;284(36):24035-48. doi: 10.1074/jbc.M109.014266. Epub 2009 Jul 15. J Biol Chem. 2009. PMID: 19605353 Free PMC article. - Neisseria gonorrhoeae activates the proteinase cathepsin B to mediate the signaling activities of the NLRP3 and ASC-containing inflammasome.
Duncan JA, Gao X, Huang MT, O'Connor BP, Thomas CE, Willingham SB, Bergstralh DT, Jarvis GA, Sparling PF, Ting JP. Duncan JA, et al. J Immunol. 2009 May 15;182(10):6460-9. doi: 10.4049/jimmunol.0802696. J Immunol. 2009. PMID: 19414800 Free PMC article. - Mitochondria: Sovereign of inflammation?
Tschopp J. Tschopp J. Eur J Immunol. 2011 May;41(5):1196-202. doi: 10.1002/eji.201141436. Eur J Immunol. 2011. PMID: 21469137 Review. - Regulation and Function of the Nucleotide Binding Domain Leucine-Rich Repeat-Containing Receptor, Pyrin Domain-Containing-3 Inflammasome in Lung Disease.
Lee S, Suh GY, Ryter SW, Choi AM. Lee S, et al. Am J Respir Cell Mol Biol. 2016 Feb;54(2):151-60. doi: 10.1165/rcmb.2015-0231TR. Am J Respir Cell Mol Biol. 2016. PMID: 26418144 Free PMC article. Review.
Cited by
- Azacytidine induces necrosis of multiple myeloma cells through oxidative stress.
Tian E, Tang H, Xu R, Liu C, Deng H, Wang Q. Tian E, et al. Proteome Sci. 2013 Jun 13;11(1):24. doi: 10.1186/1477-5956-11-24. Proteome Sci. 2013. PMID: 23764212 Free PMC article. - Inflammasomes in pancreatic physiology and disease.
Hoque R, Mehal WZ. Hoque R, et al. Am J Physiol Gastrointest Liver Physiol. 2015 Apr 15;308(8):G643-51. doi: 10.1152/ajpgi.00388.2014. Epub 2015 Feb 19. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25700081 Free PMC article. Review. - Time-Dependent Protection of CB2 Receptor Agonist in Stroke.
Yu SJ, Reiner D, Shen H, Wu KJ, Liu QR, Wang Y. Yu SJ, et al. PLoS One. 2015 Jul 17;10(7):e0132487. doi: 10.1371/journal.pone.0132487. eCollection 2015. PLoS One. 2015. PMID: 26186541 Free PMC article. - An Epigenetic Insight into NLRP3 Inflammasome Activation in Inflammation-Related Processes.
Raneros AB, Bernet CR, Flórez AB, Suarez-Alvarez B. Raneros AB, et al. Biomedicines. 2021 Nov 4;9(11):1614. doi: 10.3390/biomedicines9111614. Biomedicines. 2021. PMID: 34829842 Free PMC article. Review. - Inflammasomes in cancer: a double-edged sword.
Kolb R, Liu GH, Janowski AM, Sutterwala FS, Zhang W. Kolb R, et al. Protein Cell. 2014 Jan;5(1):12-20. doi: 10.1007/s13238-013-0001-4. Epub 2014 Jan 29. Protein Cell. 2014. PMID: 24474192 Free PMC article. Review.
References
- Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol. 2007;7:179–190. - PubMed
- Yu M, et al. HMGB1 signals through toll-like receptor (TLR) 4 and TLR2. Shock. 2006;26:174–179. - PubMed
- Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. - PubMed
- Li M, et al. An essential role of the NF-kappa B/Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128–7135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K08 AI065517/AI/NIAID NIH HHS/United States
- T32 HL007974/HL/NHLBI NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- K08AI065517/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases