Apical trafficking in epithelial cells: signals, clusters and motors - PubMed (original) (raw)
Review
Apical trafficking in epithelial cells: signals, clusters and motors
Ora A Weisz et al. J Cell Sci. 2009.
Abstract
In the early days of epithelial cell biology, researchers working with kidney and/or intestinal epithelial cell lines and with hepatocytes described the biosynthetic and recycling routes followed by apical and basolateral plasma membrane (PM) proteins. They identified the trans-Golgi network and recycling endosomes as the compartments that carried out apical-basolateral sorting. They described complex apical sorting signals that promoted association with lipid rafts, and simpler basolateral sorting signals resembling clathrin-coated-pit endocytic motifs. They also noticed that different epithelial cell types routed their apical PM proteins very differently, using either a vectorial (direct) route or a transcytotic (indirect) route. Although these original observations have generally held up, recent studies have revealed interesting complexities in the routes taken by apically destined proteins and have extended our understanding of the machinery required to sustain these elaborate sorting pathways. Here, we critically review the current status of apical trafficking mechanisms and discuss a model in which clustering is required to recruit apical trafficking machineries. Uncovering the mechanisms responsible for polarized trafficking and their epithelial-specific variations will help understand how epithelial functional diversity is generated and the pathogenesis of many human diseases.
Figures
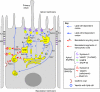
Fig. 1.
Apical trafficking routes in polarized MDCK cells. Known and suspected routes to and from apical PM domains are shown (blue arrows, blue numbers). For comparison, some of the exocytic and endocytic basolateral routes are shown (red arrows, red numbers). Rab proteins and SNAREs associated with specific routes are indicated. Route 1: lipid-raft-associated proteins, such as influenza HA, GPI-APs, sucrase-isomaltase and dipeptidylpeptidase 4, follow this route. Route 2: rhodopsin is the only protein suspected of following this route, which might be employed to reach the primary cilium. Route 3: hypothetical direct (non-transendosomal) route of raft-associated proteins to the primary cilium. This route might be used by soluble secretory proteins to avoid being targeted to the degradative pathway (Ed; endosome degradation). Route 4: the route followed by the non-raft-associated proteins p75 and sialomucin endolyn. Route 5: an apical variant of the VSVG protein is thought to pass through the CRE upon leaving the TGN. It is unknown whether subsequent transit through the ARE also occurs. Route 6: the pIgR uses a combination of basolateral sorting signals and transcytotic signals to travel first to the basolateral membrane and to then transcytose to the apical membrane via the BEE/BSE, CRE and ARE. In liver and intestinal epithelial cells, the transcytotic route is a major pathway for most apical proteins. Route 7: specific types of exosomes are exocytosed apically, suggesting the existence of apical and basolateral sorting mechanisms at late endosomes. Apical (Er; endosome recycling), degradative (Ed) and basolateral recycling (Br) routes are indicated. E, endosome; LE, late endosome; lys, lysosome; TJ, tight junction; ZA, zonula adherens.
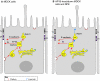
Fig. 2.
Apical localization resulting from absence of a basolateral clathrin adaptor. (A) In epithelial cells that express the clathrin adaptor AP1B (e.g. MDCK cells), basolateral proteins are recycled efficiently back to the basolateral membrane owing to the presence of this adaptor in the CRE. In these cells, basolateral proteins never reach the Rab11-positive ARE. The exception to this rule is E-cadherin, which seems to transit through a Rab11-positive compartment in its biosynthetic route (Desclozeaux et al., 2008). (B) The situation is different in non-polarized cells, in which the transferrin receptor recycles to the PM from Rab11-positive recycling endosomes. When AP1B is missing, transferrin receptors are missorted to the apical surface, probably through the ARE. This probably also happens in some native epithelia that lack AP1B, such as the RPE (Diaz et al., 2009) and renal proximal tubule (Ryan Schreiner and E.R.-B., unpublished data), although the transferrin receptor is basolaterally targeted in hepatocytes (Levine and Woods, 1990). Red arrows represent basolateral routes. Blue arrows represent an apical route that develops when AP1B is not expressed. Br, basolateral recycling route.
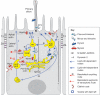
Fig. 3.
Clustering events in apical trafficking routes. Trafficking routes for the indicated cargo proteins are diagrammed. Various candidate molecules or complexes that might contribute to clustering in MDCK (A), intestinal (B) and liver (C) epithelial cells are noted within each cell type. Routes 2 (rhodopsin) and 5 (VSVG-apical variant) are hypothetical in intestinal cells. Br, basolateral recycling route; E, endocytic route; TJ, tight junction; ZA, zonula adherens.
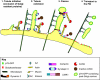
Fig. 4.
MT motors and myosins in apical trafficking. MTs are organized into two populations in polarized epithelial cells: stable cortical MTs with the minus ends facing apically, and dynamic MTs that originate at the MTOC, with the plus ends facing apically. Actin filaments have very different organizations under the apical surface (apical actin), under the lateral surface (lateral actin) and at the perinuclear region (peri-Golgi actin). The minus-end kinesin KIFC3 has been implicated in the transport of influenza HA, a lipid-raft-associated protein (route 1). Dynein participates in the transport of rhodopsin from the TGN (route 2). The plus-end kinesin KIF5B participates in the transport of p75 (route 4). Myosin-II and myosin-VI have been implicated in basolateral transport from the TGN and CRE, respectively. Myosin-IA has been implicated in post-Golgi transport, transcytosis and transport across the apical actin. E, endocytic route; Ed, endocytic degradative route; Er, endocytic recycling route.
Fig. 5.
Cooperation between the actin and MT cytoskeletons in TGN exit. Recent experiments suggest that a specialized organization of the peri-Golgi actin is regulated by LIMK1, a Golgi-resident enzyme that inactivates the actin-severing function of cofilin by phosphorylation. The short and branched actin filaments, bound to the Golgi membrane via ARF1, might be used by a Golgi myosin to bend the membrane and initiate the formation of short tubules carrying p75. The tubules are elongated by kinesin 5B, a plus-end motor, moving on the MTs. Dynamin 2, recruited via syndapin II and cortactin, participates in the fission of p75-containing vesicles and tubules that then move towards the apical surface. Modified from Salvarezza et al. (Salvarezza et al., 2008).
Similar articles
- Transcytotic efflux from early endosomes is dependent on cholesterol and glycosphingolipids in polarized hepatic cells.
Nyasae LK, Hubbard AL, Tuma PL. Nyasae LK, et al. Mol Biol Cell. 2003 Jul;14(7):2689-705. doi: 10.1091/mbc.e02-12-0816. Epub 2003 Apr 4. Mol Biol Cell. 2003. PMID: 12857857 Free PMC article. - Trafficking to the apical and basolateral membranes in polarized epithelial cells.
Stoops EH, Caplan MJ. Stoops EH, et al. J Am Soc Nephrol. 2014 Jul;25(7):1375-86. doi: 10.1681/ASN.2013080883. Epub 2014 Mar 20. J Am Soc Nephrol. 2014. PMID: 24652803 Free PMC article. Review. - Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells.
Fölsch H, Mattila PE, Weisz OA. Fölsch H, et al. Traffic. 2009 Aug;10(8):972-81. doi: 10.1111/j.1600-0854.2009.00927.x. Epub 2009 May 12. Traffic. 2009. PMID: 19453969 Free PMC article. Review. - Newly synthesized and recycling pools of the apical protein gp135 do not occupy the same compartments.
Stoops EH, Hull M, Caplan MJ. Stoops EH, et al. Traffic. 2016 Dec;17(12):1272-1285. doi: 10.1111/tra.12449. Epub 2016 Nov 1. Traffic. 2016. PMID: 27649479 Free PMC article. - Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways.
Donoso M, Cancino J, Lee J, van Kerkhof P, Retamal C, Bu G, Gonzalez A, Cáceres A, Marzolo MP. Donoso M, et al. Mol Biol Cell. 2009 Jan;20(1):481-97. doi: 10.1091/mbc.e08-08-0805. Epub 2008 Nov 12. Mol Biol Cell. 2009. PMID: 19005208 Free PMC article.
Cited by
- Controlled Plasma Membrane Delivery of FGFR1 and Modulation of Signaling by a Novel Regulated Anterograde RTK Transport Pathway.
Hinsch CL, Venkata JK, Hsu T, Dammai V. Hinsch CL, et al. Cancers (Basel). 2023 Dec 14;15(24):5837. doi: 10.3390/cancers15245837. Cancers (Basel). 2023. PMID: 38136383 Free PMC article. - The kinesin KIF16B mediates apical transcytosis of transferrin receptor in AP-1B-deficient epithelia.
Perez Bay AE, Schreiner R, Mazzoni F, Carvajal-Gonzalez JM, Gravotta D, Perret E, Lehmann Mantaras G, Zhu YS, Rodriguez-Boulan EJ. Perez Bay AE, et al. EMBO J. 2013 Jul 31;32(15):2125-39. doi: 10.1038/emboj.2013.130. Epub 2013 Jun 7. EMBO J. 2013. PMID: 23749212 Free PMC article. - Directional protein secretion by the retinal pigment epithelium: roles in retinal health and the development of age-related macular degeneration.
Kay P, Yang YC, Paraoan L. Kay P, et al. J Cell Mol Med. 2013 Jul;17(7):833-43. doi: 10.1111/jcmm.12070. Epub 2013 May 11. J Cell Mol Med. 2013. PMID: 23663427 Free PMC article. Review. - The MAL Protein, an Integral Component of Specialized Membranes, in Normal Cells and Cancer.
Rubio-Ramos A, Labat-de-Hoz L, Correas I, Alonso MA. Rubio-Ramos A, et al. Cells. 2021 Apr 30;10(5):1065. doi: 10.3390/cells10051065. Cells. 2021. PMID: 33946345 Free PMC article. Review. - Novel function for AP-1B during cell migration.
Kell MJ, Ang SF, Pigati L, Halpern A, Fölsch H. Kell MJ, et al. Mol Biol Cell. 2020 Oct 15;31(22):2475-2493. doi: 10.1091/mbc.E20-04-0256. Epub 2020 Aug 20. Mol Biol Cell. 2020. PMID: 32816642 Free PMC article.
References
- Alfalah, M., Jacob, R., Preuss, U., Zimmer, K. P., Naim, H. and Naim, H. Y. (1999). O-linked glycans mediate apical sorting of human intestinal sucrase-isomaltase through association with lipid rafts. Curr. Biol. 9, 593-596. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 GM034107/GM/NIGMS NIH HHS/United States
- DK54407/DK/NIDDK NIH HHS/United States
- EY08538/EY/NEI NIH HHS/United States
- R01 EY008538/EY/NEI NIH HHS/United States
- GM34107/GM/NIGMS NIH HHS/United States
- DK064613/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources