T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity - PubMed (original) (raw)
Comparative Study
T-cell infiltration and signaling in the adult dorsal spinal cord is a major contributor to neuropathic pain-like hypersensitivity
Michael Costigan et al. J Neurosci. 2009.
Abstract
Partial peripheral nerve injury in adult rats results in neuropathic pain-like hypersensitivity, while that in neonatal rats does not, a phenomenon also observed in humans. We therefore compared gene expression profiles in the dorsal horn of adult and neonatal rats in response to the spared nerve injury (SNI) model of peripheral neuropathic pain. The 148 differentially regulated genes in adult, but not young, rat spinal cords indicate a greater microglial and T-cell response in adult than in young animals. T-cells show a large infiltration in the adult dorsal horn but not in the neonate after SNI. T-cell-deficient Rag1-null adult mice develop less neuropathic mechanical allodynia than controls, and central expression of cytokines involved in T-cell signaling exhibits large relative differences between young and adult animals after SNI. One such cytokine, interferon-gamma (IFNgamma), is upregulated in the dorsal horn after nerve injury in the adult but not neonate, and we show that IFNgamma signaling is required for full expression of adult neuropathic hypersensitivity. These data reveal that T-cell infiltration and activation in the dorsal horn of the spinal cord following peripheral nerve injury contribute to the evolution of neuropathic pain-like hypersensitivity. The neuroimmune interaction following peripheral nerve injury has therefore a substantial adaptive immune component, which is absent or suppressed in the young CNS.
Figures
Figure 1.
Mechanical thresholds to von Frey hindpaw testing 7 d after SNI or sham surgery. Surgery was performed at P3, P10, P21, or P33. Only animals operated at P33 and adults displayed the mechanical hypersensitivity characteristic of a neuropathic pain-like response. Data are expressed as mean response threshold ± SEM. *p < 0.05, two-way ANOVA, n = 5 animals at each age.
Figure 2.
Canonical pathway analysis of signaling cascades associated genes regulated in the adult but not the neonate dorsal horn (Ad0.05/Ne0.9 gene list) using Ingenuity Pathway Analysis. Bars represent the LOD [−log(p value)] for that pathway being overrepresented within the gene list (right-tailed Fisher's exact test). LOD 1.3 (p value of 0.05) threshold used. Gene functional categories primarily representing microglial cell function and T-cell function are shown.
Figure 3.
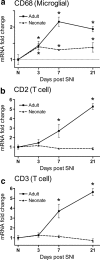
a, Quantitative PCR analysis of CD68 mRNA expression in the neonatal and adult rat dorsal horn over time after SNI relative to age-matched controls and uninjured animals, respectively. b, c, CD2 (b) and CD3 (c) transcripts increase successively over time in the adult rat dorsal horn relative to uninjured animals, but do not change in the neonatal dorsal horn relative to age-matched controls. Data are expressed as mean fold ± SEM. *p < 0.05, one-way ANOVA, Student's t test, n = 4 per group.
Figure 4.
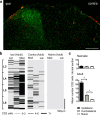
a, Lumbar dorsal horn of the rat spinal cord 7 d after SNI labeled with the microglial marker Iba1 (green) and the T-cell marker CD2 (red). Both immune cells types are predominantly present in the ipsilateral (ipsi) dorsal horn. Contra denotes contralateral. Scale bar, 250 μm. b, Distribution of T-lymphocytes in the dorsal horn of the spinal cord. Location of CD2 cells is shown as an intensity map with darker shading representing greater number of profiles per region counted. The dorsal quadrant of each 30 μm section was split into three sections: medial (Med), middle, and lateral (Lat) cord; binned cell counts were represented by a shaded bars (light gray, 0–3 cells; dark gray, 4–6 cells; black, 7 or greater), and bars were stacked relative to their position within the lumbar cord. The T-cell distribution is shown for adult ipsilateral (ipsi) and contralateral (contra) 7 d SNI and naive cord. Scale bar, 500 μm. c, Mean number ± SEM of CD2-immunoreactive cells per dorsal horn section within the L3–L6 region, in adult and neonatal ipsilateral, contralateral, and naive rats. *p < 0.05, one-way ANOVA, Bonferroni's multiple comparison, n = 3 animals per group.
Figure 5.
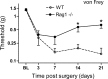
Mechanical sensitivity after SNI in _Rag1_-null animals relative to wild-type littermate controls. _Rag1_-null mice develop significantly less mechanical sensitivity in the SNI model over a 3 week time course, compared with wild-type littermate controls. All data are mean ± SEM. All values are expressed as means ± SEM. Data were analyzed using two-way ANOVA (time and genotype) for genotype (F(1,4) = 5.3; p < 0.05; post hoc Student's t test, *p < 0.05, n = 9 animals per group).
Figure 6.
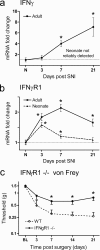
a, Expression analysis of the IFNγ mRNA by quantitative PCR reveals little evidence of expression in the injured or age-matched control neonatal dorsal horn, in contrast to the adult, where IFNγ mRNA increases successively over time. b, IFNγR1 mRNA is upregulated in the neonatal and adult dorsal horn over time after SNI; levels of regulation are, however, considerably reduced in the neonate relative to the adult. Data are expressed as mean fold ± SEM. *p < 0.05, one-way ANOVA, Student's t test, n = 4 per group. c, Mechanical sensitivity over time subsequent to SNI injury in _IFN_γ_R1_-null animals and wild-type littermate control mice. _IFN_γ_R1_-null animals display less mechanical allodynia relative to controls. All data are mean ± SEM. Data were analyzed using two-way ANOVA (time and genotype) for genotype (F(1,4) = 10.32; p < 0.05; post hoc Student's t test, *p < 0.05, n = 7 animals per group).
Similar articles
- TNF-α Differentially Regulates Synaptic Plasticity in the Hippocampus and Spinal Cord by Microglia-Dependent Mechanisms after Peripheral Nerve Injury.
Liu Y, Zhou LJ, Wang J, Li D, Ren WJ, Peng J, Wei X, Xu T, Xin WJ, Pang RP, Li YY, Qin ZH, Murugan M, Mattson MP, Wu LJ, Liu XG. Liu Y, et al. J Neurosci. 2017 Jan 25;37(4):871-881. doi: 10.1523/JNEUROSCI.2235-16.2016. J Neurosci. 2017. PMID: 28123022 Free PMC article. - Electroacupuncture Modulates Spinal BDNF/TrκB Signaling Pathway and Ameliorates the Sensitization of Dorsal Horn WDR Neurons in Spared Nerve Injury Rats.
Xue M, Sun YL, Xia YY, Huang ZH, Huang C, Xing GG. Xue M, et al. Int J Mol Sci. 2020 Sep 7;21(18):6524. doi: 10.3390/ijms21186524. Int J Mol Sci. 2020. PMID: 32906633 Free PMC article. - Low-dose methotrexate reduces peripheral nerve injury-evoked spinal microglial activation and neuropathic pain behavior in rats.
Scholz J, Abele A, Marian C, Häussler A, Herbert TA, Woolf CJ, Tegeder I. Scholz J, et al. Pain. 2008 Aug 15;138(1):130-142. doi: 10.1016/j.pain.2007.11.019. Epub 2008 Jan 22. Pain. 2008. PMID: 18215468 Free PMC article. - Sex differences in pain: a tale of two immune cells.
Mapplebeck JCS, Beggs S, Salter MW. Mapplebeck JCS, et al. Pain. 2016 Feb;157 Suppl 1:S2-S6. doi: 10.1097/j.pain.0000000000000389. Pain. 2016. PMID: 26785152 Review. - Nerve injury and neuropathic pain - A question of age.
Fitzgerald M, McKelvey R. Fitzgerald M, et al. Exp Neurol. 2016 Jan;275 Pt 2:296-302. doi: 10.1016/j.expneurol.2015.07.013. Epub 2015 Jul 26. Exp Neurol. 2016. PMID: 26220898 Free PMC article. Review.
Cited by
- Sexually Dimorphic Immune and Neuroimmune Changes Following Peripheral Nerve Injury in Mice: Novel Insights for Gender Medicine.
Vacca V, Marinelli S, De Angelis F, Angelini DF, Piras E, Battistini L, Pavone F, Coccurello R. Vacca V, et al. Int J Mol Sci. 2021 Apr 22;22(9):4397. doi: 10.3390/ijms22094397. Int J Mol Sci. 2021. PMID: 33922372 Free PMC article. - Neuropathic pain is constitutively suppressed in early life by anti-inflammatory neuroimmune regulation.
McKelvey R, Berta T, Old E, Ji RR, Fitzgerald M. McKelvey R, et al. J Neurosci. 2015 Jan 14;35(2):457-66. doi: 10.1523/JNEUROSCI.2315-14.2015. J Neurosci. 2015. PMID: 25589741 Free PMC article. - Interferon-gamma potentiates NMDA receptor signaling in spinal dorsal horn neurons via microglia-neuron interaction.
Sonekatsu M, Taniguchi W, Yamanaka M, Nishio N, Tsutsui S, Yamada H, Yoshida M, Nakatsuka T. Sonekatsu M, et al. Mol Pain. 2016 Apr 18;12:1744806916644927. doi: 10.1177/1744806916644927. Print 2016. Mol Pain. 2016. PMID: 27094552 Free PMC article. - Identification of lncRNA expression profile in the spinal cord of mice following spinal nerve ligation-induced neuropathic pain.
Jiang BC, Sun WX, He LN, Cao DL, Zhang ZJ, Gao YJ. Jiang BC, et al. Mol Pain. 2015 Jul 17;11:43. doi: 10.1186/s12990-015-0047-9. Mol Pain. 2015. PMID: 26184882 Free PMC article. - Mechanistic Differences in Neuropathic Pain Modalities Revealed by Correlating Behavior with Global Expression Profiling.
Cobos EJ, Nickerson CA, Gao F, Chandran V, Bravo-Caparrós I, González-Cano R, Riva P, Andrews NA, Latremoliere A, Seehus CR, Perazzoli G, Nieto FR, Joller N, Painter MW, Ma CHE, Omura T, Chesler EJ, Geschwind DH, Coppola G, Rangachari M, Woolf CJ, Costigan M. Cobos EJ, et al. Cell Rep. 2018 Jan 30;22(5):1301-1312. doi: 10.1016/j.celrep.2018.01.006. Cell Rep. 2018. PMID: 29386116 Free PMC article.
References
- Adkins B, Leclerc C, Marshall-Clarke S. Neonatal adaptive immunity comes of age. Nat Rev Immunol. 2004;4:553–564. - PubMed
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 DE017821/DE/NIDCR NIH HHS/United States
- G0400572/MRC_/Medical Research Council/United Kingdom
- R01 DE017821-04/DE/NIDCR NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- DE017821/DE/NIDCR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases