In vitro reconstitution of an abscisic acid signalling pathway - PubMed (original) (raw)
In vitro reconstitution of an abscisic acid signalling pathway
Hiroaki Fujii et al. Nature. 2009.
Abstract
The phytohormone abscisic acid (ABA) regulates the expression of many genes in plants; it has critical functions in stress resistance and in growth and development. Several proteins have been reported to function as ABA receptors, and many more are known to be involved in ABA signalling. However, the identities of ABA receptors remain controversial and the mechanism of signalling from perception to downstream gene expression is unclear. Here we show that by combining the recently identified ABA receptor PYR1 with the type 2C protein phosphatase (PP2C) ABI1, the serine/threonine protein kinase SnRK2.6/OST1 and the transcription factor ABF2/AREB1, we can reconstitute ABA-triggered phosphorylation of the transcription factor in vitro. Introduction of these four components into plant protoplasts results in ABA-responsive gene expression. Protoplast and test-tube reconstitution assays were used to test the function of various members of the receptor, protein phosphatase and kinase families. Our results suggest that the default state of the SnRK2 kinases is an autophosphorylated, active state and that the SnRK2 kinases are kept inactive by the PP2Cs through physical interaction and dephosphorylation. We found that in the presence of ABA, the PYR/PYL (pyrabactin resistance 1/PYR1-like) receptor proteins can disrupt the interaction between the SnRK2s and PP2Cs, thus preventing the PP2C-mediated dephosphorylation of the SnRK2s and resulting in the activation of the SnRK2 kinases. Our results reveal new insights into ABA signalling mechanisms and define a minimal set of core components of a complete major ABA signalling pathway.
Figures
Figure 1. Reconstitution of ABA signaling pathway for stress responsive gene expression in Arabidopsis protoplasts
a, SnRK2-mediated phosphorylation of ABF2 is sufficient for ABA-responsive gene expression. b, Reconstitution of ABA signaling pathway by co-expression of PYR1, ABI1, SnRK2.6 and ABF2. c, econstitution of ABA signaling pathway with different members of the PYR/PYL family. d, Reconstitution using different combinations of the core components. Protoplasts (2 × 104) from the snrk2.2/3/6 triple mutant were used except in (d), where protoplasts from the Col-0 wild-type plants were used. The RD29B::LUC and ZmUBQ::GUS were used as the ABA responsive reporter and internal control, respectively. After transfection, protoplasts were incubated for 5 h under light and in the presence of 0 (open bars) or 5 μM (solid bars) ABA. Error bars, mean ± s.e.m. (n=3).
Figure 2. ABI1 and ABI2 inhibit SnRK2.6 by dephosphorylation
a, SnRK2.6 is deactivated by ABI1. MBP or MBP-SnRK2.6 treated without (−) or with GST-ABI1 or GST was incubated with GST-ABF2 fragment (amino acids Gly73 to Gln119) in the presence of [γ32P]-ATP. In the furthest right lane (post), GST-ABI1 was added after phosphorylation of GST-ABF2 fragment by MBP-SnRK2.6. Bands of GST-ABF2 fragment and MBP-SnRK2.6 are indicated by an arrow and an arrowhead, respectively. Radioactivities of GST-ABF2 fragment bands were measured with a phospho-imager and were normalized, taking the radioactivity of the band by MBP-SnRK2.6 without ABI1 treatment as 1 (mean ± s.e.m., n = 5). b, Coomassie staining of purified MBP, SnRK2.6, ABF2, GST and GST-ABI1. c, FLAG-SnRK2.6 extracted from transgenic plants before and after ABA treatment was used instead of MBP-SnRK2.6 in (a). Coomassie staining, autoradiography and relative radioactivities (mean ± s.e.m. n = 5) of GST-ABF2 fragment are shown. Western blotting with anti-FLAG antibody shows FLAG-SnRK2.6 protein amount. d, Autoradiography of autophosphorylated SnRK2.6 showing dephosphorylation of SnRK2.6 by MBP-ABI1 and MBP-ABI2 and the effect of PYL8 and PYL5, respectively, in the presence of 1 μM ABA. e, Phosphate release from synthetic peptide HSQPKpSTVGTP, corresponding to amino acids 170-180 of SnRK2.6.
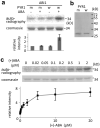
Figure 3. The combined effect of ABA, PYR1 and ABI1 on SnRK2.6 phosphorylation of GST-ABF2 fragment in vitro
a, Reconstitution of ABA regulation of ABF2 phosphorylation. MBP-SnRK2.6 treated with GST-ABI1 and His-tagged wild type PYR1 (w) or mutated PYR1P88S (m) in the absence (−) or presence (+) of 2 μM (+)-ABA was incubated with GST-ABF2 fragment (amino acids Gly73 to Gln119) in the presence of [γ32P]-ATP. Coomassie staining, autoradiography and relative radioactivities of GST-ABF2 fragment are shown. Radioactivities of GST-ABF2 fragment were normalized, taking the radioactivity of the band with PYR1P88S in the absence of ABA as 1 (mean ± s.e.m., n = 5). b, Coomassie staining of PYR1 (w) and PYR1P88S (m). c, ABA dose response. MBP-SnRK2.6, GST-ABI1 and His-PYR1 were incubated with different concentrations of (+)-ABA before the kinase assay using GST-ABF2 fragment as substrate. Coomassie staining, autoradiography and relative radioactivities (taking the radioactivity of the band in the absence of ABA as 1; mean ± s.e.m., n = 9 for < 5 μM, n = 4 for 5 μM or more) of GST-ABF2 fragment are shown.
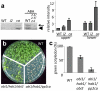
Figure 4. Effect of PP2C mutations on ABA response phenotypes and kinase activities of SnRK2s
a, In-gel kinase assay showing the activities of SnRK2s in the abi1/hab1/abi2 (i2) and abi1/hab1/pp2ca (ca) triple mutants. The snrk2.2/2.3/2.6 was used as a control. GST-fused ABF2 fragment (amino acids Gly73 to Gln119) was used as the phosphorylation substrate. The expected positions of SnRK2.6 and SnRK2.2/2.3 are indicated by arrow and arrowhead, respectively. Radioactivities of the upper and lower bands were normalized, taking the radioactivity of the upper band in WT as 1 (mean ± s.e.m., n = 3). b, The PP2C triple mutants show ABA hypersensitivity during germination and early seedling development. Photograph of plants of the indicated genotypes growing on MS medium with 3% sucrose for 14 d. c, The percentage of seedlings with green cotyledons 6 d after the end of stratification is shown (mean ± s.e.m., n = 3).
Comment in
- Plant biology: Signal advance for abscisic acid.
Sheard LB, Zheng N. Sheard LB, et al. Nature. 2009 Dec 3;462(7273):575-6. doi: 10.1038/462575a. Nature. 2009. PMID: 19956245 No abstract available.
Similar articles
- PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis.
Nishimura N, Sarkeshik A, Nito K, Park SY, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, Yates JR, Schroeder JI. Nishimura N, et al. Plant J. 2010 Jan;61(2):290-9. doi: 10.1111/j.1365-313X.2009.04054.x. Epub 2009 Oct 26. Plant J. 2010. PMID: 19874541 Free PMC article. - Reconstitution of Abscisic Acid Signaling from the Receptor to DNA via bHLH Transcription Factors.
Takahashi Y, Ebisu Y, Shimazaki KI. Takahashi Y, et al. Plant Physiol. 2017 Jun;174(2):815-822. doi: 10.1104/pp.16.01825. Epub 2017 Apr 24. Plant Physiol. 2017. PMID: 28438792 Free PMC article. - Osmotic signaling releases PP2C-mediated inhibition of Arabidopsis SnRK2s via the receptor-like cytoplasmic kinase BIK1.
Li GJ, Chen K, Sun S, Zhao Y. Li GJ, et al. EMBO J. 2024 Dec;43(23):6076-6103. doi: 10.1038/s44318-024-00277-0. Epub 2024 Oct 21. EMBO J. 2024. PMID: 39433899 Free PMC article. - Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants.
Fujita Y, Yoshida T, Yamaguchi-Shinozaki K. Fujita Y, et al. Physiol Plant. 2013 Jan;147(1):15-27. doi: 10.1111/j.1399-3054.2012.01635.x. Epub 2012 May 16. Physiol Plant. 2013. PMID: 22519646 Review. - Early abscisic acid signal transduction mechanisms: newly discovered components and newly emerging questions.
Hubbard KE, Nishimura N, Hitomi K, Getzoff ED, Schroeder JI. Hubbard KE, et al. Genes Dev. 2010 Aug 15;24(16):1695-708. doi: 10.1101/gad.1953910. Genes Dev. 2010. PMID: 20713515 Free PMC article. Review.
Cited by
- Linking Brassinosteroid and ABA Signaling in the Context of Stress Acclimation.
Bulgakov VP, Avramenko TV. Bulgakov VP, et al. Int J Mol Sci. 2020 Jul 20;21(14):5108. doi: 10.3390/ijms21145108. Int J Mol Sci. 2020. PMID: 32698312 Free PMC article. Review. - Rice OsPUB16 modulates the 'SAPK9-OsMADS23-OsAOC' pathway to reduce plant water-deficit tolerance by repressing ABA and JA biosynthesis.
Lv Q, Li X, Jin X, Sun Y, Wu Y, Wang W, Huang J. Lv Q, et al. PLoS Genet. 2022 Nov 28;18(11):e1010520. doi: 10.1371/journal.pgen.1010520. eCollection 2022 Nov. PLoS Genet. 2022. PMID: 36441771 Free PMC article. - Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability.
Mickelbart MV, Hasegawa PM, Bailey-Serres J. Mickelbart MV, et al. Nat Rev Genet. 2015 Apr;16(4):237-51. doi: 10.1038/nrg3901. Epub 2015 Mar 10. Nat Rev Genet. 2015. PMID: 25752530 Review. - Post-translational control of ABA signalling: the roles of protein phosphorylation and ubiquitination.
Yang W, Zhang W, Wang X. Yang W, et al. Plant Biotechnol J. 2017 Jan;15(1):4-14. doi: 10.1111/pbi.12652. Epub 2016 Dec 6. Plant Biotechnol J. 2017. PMID: 27767245 Free PMC article. Review. - The maize OST1 kinase homolog phosphorylates and regulates the maize SNAC1-type transcription factor.
Vilela B, Moreno-Cortés A, Rabissi A, Leung J, Pagès M, Lumbreras V. Vilela B, et al. PLoS One. 2013;8(2):e58105. doi: 10.1371/journal.pone.0058105. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23469147 Free PMC article.
References
- Hetherington AM. Guard cell signaling. Cell. 2001;107:711–714. - PubMed
- Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signalling and engineering of drought hardiness in plants. Nature. 2001;410:327–330. - PubMed
- Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Curr. Opin. Plant Biol. 2002;5:33–36. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases