Cerebral and spinal modulation of pain by emotions - PubMed (original) (raw)
Clinical Trial
. 2009 Dec 8;106(49):20900-5.
doi: 10.1073/pnas.0904706106. Epub 2009 Nov 19.
Affiliations
- PMID: 19926861
- PMCID: PMC2779826
- DOI: 10.1073/pnas.0904706106
Clinical Trial
Cerebral and spinal modulation of pain by emotions
Mathieu Roy et al. Proc Natl Acad Sci U S A. 2009.
Abstract
Emotions have powerful effects on pain perception. However, the brain mechanisms underlying these effects remain largely unknown. In this study, we combined functional cerebral imaging with psychophysiological methods to explore the neural mechanisms involved in the emotional modulation of spinal nociceptive responses (RIII-reflex) and pain perception in healthy participants. Emotions induced by pleasant or unpleasant pictures modulated the responses to painful electrical stimulations in the right insula, paracentral lobule, parahippocampal gyrus, thalamus, and amygdala. Right insula activation covaried with the modulation of pain perception, consistent with a key role of this structure in the integration of pain signals with the ongoing emotion. In contrast, activity in the thalamus, amygdala, and several prefrontal areas was associated with the modulation of spinal reflex responses. Last, connectivity analyses suggested an involvement of prefrontal, parahippocampal, and brainstem structures in the cerebral and cerebrospinal modulation of pain by emotions. This multiplicity of mechanisms underlying the emotional modulation of pain is reflective of the strong interrelations between pain and emotions, and emphasizes the powerful effects that emotions can have on pain.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Fig. 1.
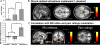
Experimental paradigm and brain responses to painful shocks and pictures. (A) Each trial consists of a block of five 6-s pictures (neutral, pleasant, or unpleasant), within which two painful electrical stimulations are delivered 2,700 ms after the onset of the 2nd and 4th pictures. At the end of the trial, participants were asked to rate the mean pain elicited by the two painful stimulation on a VAS. (B) Electrical stimulations were delivered over the right sural nerve (ankle) and RIII reflexes were extracted from the EMG of the biceps femoris in a window of 90–180 ms after the onset of the electrical stimulation. (C) A mixed block/event-related model was used to analyze the data. Pictures were modeled as blocks and electrical stimulations as events. (D) Electrical stimulations elicited activations in the thalamus (Thal), hypothalamus (Hyp), SI/SII, ACC, insula (Ins), precentral gyrus (PCG), SMA, OFC, and cerebellum (Cb). Pictures elicited activation in visual areas such as the inferior occipital gyrus (IOG) and fusiform gyrus (FFG) (see
Table S1
).
Fig. 2.
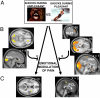
Modulation of pain-related responses by the emotional conditions. (A) Effects of the experimental condition on pain ratings (Upper). Effects of the experimental condition on the standardised R-III reflex amplitudes (Lower). *, P < 0.05. (B) Regions where shock-related activity was higher during the viewing of unpleasant compared with pleasant pictures included the PCL, right Ins, and bilateral PHG. (C) Regions where the amplitude of the modulation of brain responses between unpleasant and pleasant pictures correlated with the corresponding modulation in RIII reflex or pain ratings. (Left) Correlations with RIII reflex modulation included the left medial Thal, left pons, bilateral amygdalae (Amy), SgCC, VMPFC and MPFC, and Cb. (Right) Correlations with pain modulation (pain ratings) included the right Ins and bilateral lingual gyri (LG). Activation maps are thresholded at P < 0.005 for display purposes (see
Tables S2 and S3
).
Fig. 3.
Results of the PPI analyses. (A) The psychological variable for the interaction is the contrast between the electrical stimulations presented during unpleasant vs. pleasant pictures. (B) Right OFC, SgCC, and cuneus (Cun) exhibited higher connectivity with the right insula for the electrical stimulations presented during the viewing of unpleasant vs. pleasant pictures (Left). Left DLPFC, FFG, and PHG, left SMA, left Thal, and RM showed higher connectivity with the PCL for the electrical stimulations presented during the viewing of unpleasant vs. pleasant pictures (Right). (C) The right Ins and the PCL, where activation to electrical stimulations were shown to be higher during unpleasant than pleasant pictures, served as the physiological variables (“seeds”) for the PPIs. Activation maps were thresholded at P < 0.005 for display purposes (see
Table S5
).
Similar articles
- The modulation of pain by attention and emotion: a dissociation of perceptual and spinal nociceptive processes.
Roy M, Lebuis A, Peretz I, Rainville P. Roy M, et al. Eur J Pain. 2011 Jul;15(6):641.e1-10. doi: 10.1016/j.ejpain.2010.11.013. Epub 2010 Dec 31. Eur J Pain. 2011. PMID: 21196127 - The effect of emotion regulation on the emotional modulation of pain and nociceptive flexion reflex.
Toledo TA, Vore CN, Huber FA, Rhudy JL. Toledo TA, et al. Pain. 2024 Jun 1;165(6):1266-1277. doi: 10.1097/j.pain.0000000000003127. Epub 2024 Jan 26. Pain. 2024. PMID: 38227556 - Different Brain Circuitries Mediating Controllable and Uncontrollable Pain.
Bräscher AK, Becker S, Hoeppli ME, Schweinhardt P. Bräscher AK, et al. J Neurosci. 2016 May 4;36(18):5013-25. doi: 10.1523/JNEUROSCI.1954-15.2016. J Neurosci. 2016. PMID: 27147654 Free PMC article. - [Cerebral mechanisms involved in the interaction between pain and emotion].
Duquette M, Roy M, Leporé F, Peretz I, Rainville P. Duquette M, et al. Rev Neurol (Paris). 2007 Feb;163(2):169-79. doi: 10.1016/s0035-3787(07)90388-4. Rev Neurol (Paris). 2007. PMID: 17351536 Review. French. - [Involvement of amygdala in the emotional processing of pain].
Huang J, Kang XZ, Luo P. Huang J, et al. Sheng Li Ke Xue Jin Zhan. 2005 Oct;36(4):289-94. Sheng Li Ke Xue Jin Zhan. 2005. PMID: 16408764 Review. Chinese.
Cited by
- Is blood glucose associated with descending modulation of spinal nociception as measured by the nociceptive flexion reflex?
Terry EL, Güereca YM, Martin SL, Rhudy JL. Terry EL, et al. J Pain Res. 2016 Apr 4;9:187-93. doi: 10.2147/JPR.S101720. eCollection 2016. J Pain Res. 2016. PMID: 27110138 Free PMC article. - Thalamocortical Mechanisms for Nostalgia-Induced Analgesia.
Zhang M, Yang Z, Zhong J, Zhang Y, Lin X, Cai H, Kong Y. Zhang M, et al. J Neurosci. 2022 Apr 6;42(14):2963-2972. doi: 10.1523/JNEUROSCI.2123-21.2022. Epub 2022 Mar 1. J Neurosci. 2022. PMID: 35232762 Free PMC article. - Brain activity associated with illusory correlations in animal phobia.
Wiemer J, Schulz SM, Reicherts P, Glotzbach-Schoon E, Andreatta M, Pauli P. Wiemer J, et al. Soc Cogn Affect Neurosci. 2015 Jul;10(7):969-77. doi: 10.1093/scan/nsu142. Epub 2014 Nov 18. Soc Cogn Affect Neurosci. 2015. PMID: 25411452 Free PMC article. - Can positive affect attenuate (persistent) pain? State of the art and clinical implications.
Hanssen MM, Peters ML, Boselie JJ, Meulders A. Hanssen MM, et al. Curr Rheumatol Rep. 2017 Nov 9;19(12):80. doi: 10.1007/s11926-017-0703-3. Curr Rheumatol Rep. 2017. PMID: 29119260 Free PMC article. Review.
References
- Price DD. Psychological and neural mechanisms of the affective dimension of pain. Science. 2000;288:1769–1772. - PubMed
- Villemure C, Bushnell MC. Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain. 2002;95:195–199. - PubMed
- Rhudy JL, Williams AE, McCabe KM, Nguyen MATV, Rambo P. Affective modulation of nociception at spinal and supraspinal levels. Psychophysiology. 2005;42:579–587. - PubMed
- Lang PJ, Bradley MM, Cuthbert BN. International affective pictures system (IAPS): Digitized photographs, instruction manual and affective ratings. Gainesville: University of Florida, NIMH Center for the Study of Emotion and Attention; 1998. (Tech. Rep. A-6)
- Sandrini G, et al. The lower limb flexion reflex in humans. Prog Neurobiol. 2005;77:353–395. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical