Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy - PubMed (original) (raw)
Randomized Controlled Trial
. 2009 Dec;20(12):2641-50.
doi: 10.1681/ASN.2009070737. Epub 2009 Nov 19.
Affiliations
- PMID: 19926893
- PMCID: PMC2794224
- DOI: 10.1681/ASN.2009070737
Randomized Controlled Trial
Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy
Uzma F Mehdi et al. J Am Soc Nephrol. 2009 Dec.
Abstract
Aldosterone promotes glomerular and tubular sclerosis independent of angiotensin II in animal models of diabetic nephropathy. Most human studies testing the renoprotective benefit of adding an angiotensin receptor blocker or a mineralocorticoid receptor antagonist to a regimen based on inhibition of angiotensin-converting enzyme (ACE) used relatively low doses of ACE inhibitors. Furthermore, these studies did not determine whether antiproteinuric effects were independent of BP lowering. We conducted a double-blind, placebo-controlled trial in 81 patients with diabetes, hypertension, and albuminuria (urine albumin-to-creatinine ratio > or =300 mg/g) who all received lisinopril (80 mg once daily). We randomly assigned the patients to placebo, losartan (100 mg daily), or spironolactone (25 mg daily) for 48 wk. We obtained blood and urine albumin, urea, creatinine, electrolytes, A1c, and ambulatory BP at baseline, 24, and 48 wk. Compared with placebo, the urine albumin-to-creatinine ratio decreased by 34.0% (95% CI, -51.0%, -11.2%, P = 0.007) in the group assigned to spironolactone and by 16.8% (95% CI, -37.3%, +10.5%, P = 0.20) in the group assigned to losartan. Clinic and ambulatory BP, creatinine clearance, sodium and protein intake, and glycemic control did not differ between groups. Serum potassium level was significantly higher with the addition of either spironolactone or losartan. In conclusion, the addition of spironolactone, but not losartan, to a regimen including maximal ACE inhibition affords greater renoprotection in diabetic nephropathy despite a similar effect on BP. These results support the need to conduct a long-term, large-scale, renal failure outcomes trial.
Figures
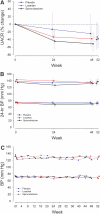
Figure 1.
The UACR and BP treatment responses for each study group. Solid lines indicate active treatment weeks, and the dotted lines indicate the washout phase. (A) The UACR percentage change for spironolactone versus placebo (−34.0%, 95% CI, −51.0%, −11.2%, P = 0.007) and losartan versus placebo (95% CI, −37.3%, +10.5%, P = 0.2). Data are presented as the percentage change from the baseline geometric mean and 95% CI. The P values are from mixed-model repeated-measures analysis. See the text for within-group differences. (B) Twenty-four-hour systolic BP percentage change was not statistically different between groups. Data are presented as the mean and 95% CI. (C) Clinical BP responses were not statistically different between groups. Data are presented as the mean and 95% CI.
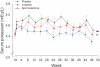
Figure 2.
Serum potassium treatment response by study group. Serum potassium for losartan versus placebo, P = 0.03; spironolactone versus placebo, P < 0.0001; losartan versus spironolactone, P = 0.05. Solid lines indicate active treatment weeks, and the dotted lines indicate the washout phase. Data are presented as the mean and standard error.
Figure 3.
Study Design. Asterisks denote inpatient CTRC admission.
Figure 4.
Number of study subjects who were screened, enrolled, randomized, and completed the study.
Comment in
- Aldosterone blockade in diabetic nephropathy: relative risks and potential promise.
Rubin MF, Townsend RR. Rubin MF, et al. J Am Soc Nephrol. 2009 Dec;20(12):2487-9. doi: 10.1681/ASN.2009101036. Epub 2009 Oct 29. J Am Soc Nephrol. 2009. PMID: 19875814 Review. No abstract available. - Use of medications to lower urine protein level in patients with diabetic kidney disease.
Stanton RC. Stanton RC. Curr Diab Rep. 2010 Aug;10(4):257-60. doi: 10.1007/s11892-010-0125-3. Curr Diab Rep. 2010. PMID: 20532702 No abstract available.
Similar articles
- Potassium handling with dual renin-angiotensin system inhibition in diabetic nephropathy.
Van Buren PN, Adams-Huet B, Nguyen M, Molina C, Toto RD. Van Buren PN, et al. Clin J Am Soc Nephrol. 2014 Feb;9(2):295-301. doi: 10.2215/CJN.07460713. Epub 2014 Jan 9. Clin J Am Soc Nephrol. 2014. PMID: 24408116 Free PMC article. Clinical Trial. - Beneficial impact of spironolactone on nephrotic range albuminuria in diabetic nephropathy.
Schjoedt KJ, Rossing K, Juhl TR, Boomsma F, Tarnow L, Rossing P, Parving HH. Schjoedt KJ, et al. Kidney Int. 2006 Aug;70(3):536-42. doi: 10.1038/sj.ki.5001580. Epub 2006 Jun 14. Kidney Int. 2006. PMID: 16775595 Clinical Trial. - Renoprotective effect of the addition of losartan to ongoing treatment with an angiotensin converting enzyme inhibitor in type-2 diabetic patients with nephropathy.
Abe H, Minatoguchi S, Ohashi H, Murata I, Minagawa T, Okuma T, Yokoyama H, Takatsu H, Takaya T, Nagano T, Osumi Y, Kakami M, Tsukamoto T, Tanaka T, Hiei K, Fujiwara H. Abe H, et al. Hypertens Res. 2007 Oct;30(10):929-35. doi: 10.1291/hypres.30.929. Hypertens Res. 2007. PMID: 18049024 Clinical Trial. - Emerging trends for prevention and treatment of diabetic nephropathy: blockade of the RAAS and BP control.
Hunsicker LG. Hunsicker LG. J Manag Care Pharm. 2004 Sep;10(5 Suppl A):S12-7. doi: 10.18553/jmcp.2004.10.S5-A.S12. J Manag Care Pharm. 2004. PMID: 15369420 Free PMC article. Review.
Cited by
- Renin-Angiotensin-Aldosterone System Modulators in Adults with Hypertension: A Network Meta-Analysis of Randomized Controlled Trials.
Yi X, Yang S, Yang J, Chen X, Zhang A, Zeng Q, Luo W, Li Q, Hu J. Yi X, et al. Drugs. 2024 Sep 23. doi: 10.1007/s40265-024-02092-7. Online ahead of print. Drugs. 2024. PMID: 39312177 - Mineralocorticoid receptor overactivation in diabetes mellitus: role of O-GlcNAc modification.
Jo R, Itoh H, Shibata H. Jo R, et al. Hypertens Res. 2024 Aug;47(8):2126-2132. doi: 10.1038/s41440-024-01734-3. Epub 2024 May 24. Hypertens Res. 2024. PMID: 38789539 Review. - Angiotensin-converting-enzyme inhibitors and angiotensin receptor blockers for preventing the progression of diabetic kidney disease.
Natale P, Palmer SC, Navaneethan SD, Craig JC, Strippoli GF. Natale P, et al. Cochrane Database Syst Rev. 2024 Apr 29;4(4):CD006257. doi: 10.1002/14651858.CD006257.pub2. Cochrane Database Syst Rev. 2024. PMID: 38682786 - Finerenone: From the Mechanism of Action to Clinical Use in Kidney Disease.
Piko N, Bevc S, Hojs R, Ekart R. Piko N, et al. Pharmaceuticals (Basel). 2024 Mar 26;17(4):418. doi: 10.3390/ph17040418. Pharmaceuticals (Basel). 2024. PMID: 38675379 Free PMC article. Review. - Efficacy and safety of nonsteroidal mineralocorticoid receptor antagonists for renal and cardiovascular outcomes in patients with chronic kidney disease: a meta-analysis of randomized clinical trials.
Chen Q, Wei G, Wang Y, Li X, Zhao Q, Zhu L, Xiao Q, Xiong X. Chen Q, et al. Front Pharmacol. 2024 Feb 27;15:1338044. doi: 10.3389/fphar.2024.1338044. eCollection 2024. Front Pharmacol. 2024. PMID: 38476327 Free PMC article.
References
- Adler S: Diabetic nephropathy: Linking histology, cell biology, and genetics. Kidney Int 66: 2095–2106, 2004 - PubMed
- Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR: Development and progression of nephropathy in type 2 diabetes: The United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63: 225–232, 2003 - PubMed
- Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 - PubMed
- Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O: Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348: 383–393, 2003 - PubMed
- Andersen S, Tarnow L, Rossing P, Hansen BV, Parving HH: Renoprotective effects of angiotensin II receptor blockade in type 1 diabetic patients with diabetic nephropathy. Kidney Int 57: 601–606, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2R01 DK6301001/DK/NIDDK NIH HHS/United States
- M01-RR-00633/RR/NCRR NIH HHS/United States
- M01 RR000633/RR/NCRR NIH HHS/United States
- UL1 RR024982/RR/NCRR NIH HHS/United States
- UL1-RR-024982/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous