Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex - PubMed (original) (raw)
Gene loops function to maintain transcriptional memory through interaction with the nuclear pore complex
Sue Mei Tan-Wong et al. Genes Dev. 2009.
Abstract
Inducible genes in yeast retain a "memory" of recent transcriptional activity during periods of short-term repression, allowing them to be reactivated faster when reinduced. This confers a rapid and versatile gene expression response to the environment. We demonstrate that this memory mechanism is associated with gene loop interactions between the promoter and 3' end of the responsive genes HXK1 and GAL1FMP27. The maintenance of these memory gene loops (MGLs) during intervening periods of transcriptional repression is required for faster RNA polymerase II (Pol II) recruitment to the genes upon reinduction, thereby facilitating faster mRNA accumulation. Notably, a sua7-1 mutant or the endogenous INO1 gene that lacks this MGL does not display such faster reinduction. Furthermore, these MGLs interact with the nuclear pore complex through association with myosin-like protein 1 (Mlp1). An mlp1Delta strain does not maintain MGLs, and concomitantly loses transcriptional memory. We predict that gene loop conformations enhance gene expression by facilitating rapid transcriptional response to changing environmental conditions.
Figures
Figure 1.
Mlp1 is required for gene loop maintenance during glucose shutdown. (A) Schematic of HXK1 indicating positions of 3C primers (above) and ChIP probes (below). Vertical lines depict MseI restriction sites used in 3C analysis (thicker lines depict very closely spaced restriction sites). (B) RT–qPCR analysis of HXK1 mRNA kinetic response to galactose induction and glucose repression. (C) Pol II ChIP analysis on HXK1 in wild type and mlp1Δ during a glucose shutdown time course. A–D refer to positions along the gene as indicated in A. Values are shown as a ratio of time point 0 min′. (D) HXK1 time-course 3C analysis on wild-type, mlp1Δ, and nup2Δ strains during a glucose shutdown time course. (Left panel) Short time course. (Right panel) A similar experiment including longer time points. 3C was performed using the primers indicated in A. (E) Mlp1 ChIP analysis on HXK1. (Left) Mlp1-GFP ChIP analysis on HXK1 at UAS, promoter, ORF, and 3′-end locations (as indicated in A) under overnight glucose (GLU) and galactose (GAL) conditions. The GAL1 UAS was a positive control (Luthra et al. 2007). Values are normalized to GLU conditions. (Right) Mlp1-GFP ChIP on HXK1 at 60 min and 4.5 h of glucose repression. Values are shown as immunoprecipitation/input after normalization to a non-GFP-tagged control strain. Values approaching 1 reflect background signals.
Figure 2.
Mlp1-facilitated gene loops are required for transcriptional memory in HXK1. (A) Schematic of galactose induction and reinduction time course used for the following data sets. (B) HXK1 RT–qPCR (top panel), Pol II ChIP (middle panel), and 3C (bottom panel) data showing comparative kinetics between wild type (left panel) and mlp1Δ (right panel) under the culture conditions described in A. 3C primers are the same as in Figure 1A. The primer pair used for RT–qPCR as well as the ChIP probe is denoted “C” in Figure 1A.
Figure 3.
Mlp1-facilitated gene loops are required for fast transcriptional memory in GAL1∷FMP27. (A) Schematic of GAL1∷FMP27 indicating positions of 3C primers (above) and ChIP probe (below). Vertical lines depict EcoRI restriction sites used in 3C analysis. (B) GAL1∷FMP27 RT–qPCR (top panel), Pol II ChIP (middle panel), and 3C (bottom panel) data showing comparative kinetics between wild type (left panel) and mlp1Δ (right panel) under culture conditions described in Figure 2A. (C) GAL1∷FMP27 RT–qPCR time course of wild-type and mlp1Δ strains reinduced after a very short 5-min period of intervening repression (during which the MGL is still present, even in mlp1Δ). These data are an average of multiple biological repeats. Error bars have been included but are very small, so not clearly visible.
Figure 4.
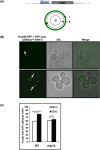
Mlp1 is required for HXK1 localization to the NPC upon activation. (A, top) Schematic of yeast cells with 256 _lac_op repeats upstream of HXK1 and expressing LacI-GFP and Nup49-GFP fusions (Taddei et al. 2006). (Bottom) Schematic of the nuclear focal plane with the Nup49-GFP-labeled nuclear periphery and categorization of zones I, II, and III. (B) Representative microscopy analysis of the LacI-GFP-HXK1 dot in subnuclear positions of zone III (top) and zone I (bottom). (C) Percentage of cells with LacI-GFP-HXK1 tethered to the NPC upon galactose activation in wild type and mlp1Δ.
Figure 5.
Disrupted gene looping in sua7-1 causes loss of NPC tethering and transcriptional memory. (A) HXK1 3C gene loop analysis of sua7-1 and its isogenic wild type at time points of overnight glucose repression (GLU o/n), active galactose transcription (GAL 60′), and a subsequent 60-min glucose repression (GLU 60′). The plus sign (+) refers to the 3C PCR-positive control (see Supplemental Fig. 1C for details). 3C primers are the same as in Figure 1A. (B, top) Schematic of galactose induction and reinduction time course used for the following RT–qPCR data sets. (Bottom) RT–qPCR data showing comparative naïve induction and second-round reinduction kinetics in wild type. (C) RT–qPCR data showing comparative reinduction kinetics between wild type and sua7-1. (D) Mlp1 ChIP on HXK1 performed on GFP-tagged Mlp1 in wild-type and sua7-1 strains during galactose induction and a subsequent 60-min glucose repression (memory condition).
Figure 6.
Absence of repression gene loop in INO1 correlates with lack of transcriptional memory. (A) Schematic of INO1 indicating positions of 3C primers. Vertical lines depict HaeIII restriction sites used in 3C analysis. (B) Schematic of the ±100 μM inositol induction/repression time course used for C and D. (C) INO1 3C data showing the kinetics of INO1 loop presence under the culture conditions described in B. (3C primers) I2–I6 as depicted in A. The black arrow points to the 1-h period of intervening repression during which an MGL would predictably be retained. (D) INO1 RT-qPCR data showing the kinetics of INO1 induction and reinduction under the culture conditions described in B. (E) Mlp1 ChIP on INO1 (on an Mlp1-GFP-tagged strain). The schematic at the top details the four time points at which the ChIPs were performed. Mlp1 ChIP at the GAL1 UAS was performed as a positive control for the ChIP (Luthra et al. 2007). As such, the time course was performed under differing glucose and galactose conditions (as indicated in the schematic) in order to ascertain Mlp1 presence on GAL1 UAS during active galactose conditions, and its absence during repressive glucose conditions.
Figure 7.
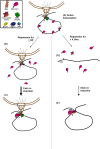
Model for how Mlp1-facilitated gene loops function to retain transcriptional memory. (A) Elongation and active transcription during inductive growth conditions, with the gene tethered to the NPC in a loop confirmation. (B) Repression for ≤60 min: The gene loop is retained at the NPC through Mlp1 involvement despite Pol II leaving the template. (C) Upon reinduction, Pol II is able to load onto the promoter and begin faster transcription faster due to retention of transcription factors in the loop scaffold. This causes the faster reinduction memory effects with consequent faster mRNA accumulation. (D) With repression for ≥4.5 h, gene loop conformation as well as the NPC association is eventually lost; certain transcription factors are released due to a lack of the loop scaffold. (E) Upon reinduction, the transcription initiation complex forms de novo, resulting in comparatively slower kinetics of transcription reinduction. Gene loops reform upon transcription and the gene then becomes tethered to the NPC as in A and C. Protein factors potentially involved in the loop scaffold are depicted in the schematic legend: Mex67 is known to be recruited to the promoter regions of GAL10 and HSP104 during gene activation and promotes gene anchoring through its association with Mlp1 (Dieppois et al. 2006). SAGA is recruited to the GAL UAS by Gal4 during galactose activation and SAGA complex proteins interact with Mlp1 at active genes, an interaction required for Mlp1 association with the GAL promoter (Luthra et al. 2007). SAGA-associated Sus1, a component of the mRNA export complex TREX-2, is necessary for nuclear peripheral positioning of active genes, and binds the NPC through Nup1 (Fischer et al. 2002; Rodriguez-Navarro et al. 2004; Cabal et al. 2006; Drubin et al. 2006; Chekanova et al. 2008). Nup2 interacts with promoters of active genes (Schmid et al. 2006).
Similar articles
- A physiological role for gene loops in yeast.
Lainé JP, Singh BN, Krishnamurthy S, Hampsey M. Lainé JP, et al. Genes Dev. 2009 Nov 15;23(22):2604-9. doi: 10.1101/gad.1823609. Genes Dev. 2009. PMID: 19933150 Free PMC article. - Interaction of a DNA zip code with the nuclear pore complex promotes H2A.Z incorporation and INO1 transcriptional memory.
Light WH, Brickner DG, Brand VR, Brickner JH. Light WH, et al. Mol Cell. 2010 Oct 8;40(1):112-25. doi: 10.1016/j.molcel.2010.09.007. Mol Cell. 2010. PMID: 20932479 Free PMC article. - Evidence for a complex of transcription factor IIB with poly(A) polymerase and cleavage factor 1 subunits required for gene looping.
Medler S, Al Husini N, Raghunayakula S, Mukundan B, Aldea A, Ansari A. Medler S, et al. J Biol Chem. 2011 Sep 30;286(39):33709-18. doi: 10.1074/jbc.M110.193870. Epub 2011 Aug 11. J Biol Chem. 2011. PMID: 21835917 Free PMC article. - Control of eukaryotic gene expression: gene loops and transcriptional memory.
Hampsey M, Singh BN, Ansari A, Lainé JP, Krishnamurthy S. Hampsey M, et al. Adv Enzyme Regul. 2011;51(1):118-25. doi: 10.1016/j.advenzreg.2010.10.001. Epub 2010 Oct 29. Adv Enzyme Regul. 2011. PMID: 21036187 Free PMC article. Review. - Nuclear pore proteins regulate chromatin structure and transcriptional memory by a conserved mechanism.
Light WH, Brickner JH. Light WH, et al. Nucleus. 2013 Sep-Oct;4(5):357-60. doi: 10.4161/nucl.26209. Epub 2013 Aug 19. Nucleus. 2013. PMID: 23962805 Free PMC article. Review.
Cited by
- AP-1 Mediates Cellular Adaptation and Memory Formation During Therapy Resistance.
Li J, Ravindran PT, O'Farrell A, Busch GT, Boe RH, Niu Z, Woo S, Dunagin MC, Jain N, Goyal Y, Sarma K, Herlyn M, Raj A. Li J, et al. bioRxiv [Preprint]. 2024 Jul 25:2024.07.25.604999. doi: 10.1101/2024.07.25.604999. bioRxiv. 2024. PMID: 39091739 Free PMC article. Preprint. - A physiological role for gene loops in yeast.
Lainé JP, Singh BN, Krishnamurthy S, Hampsey M. Lainé JP, et al. Genes Dev. 2009 Nov 15;23(22):2604-9. doi: 10.1101/gad.1823609. Genes Dev. 2009. PMID: 19933150 Free PMC article. - The dynamic architectural and epigenetic nuclear landscape: developing the genomic almanac of biology and disease.
Tai PW, Zaidi SK, Wu H, Grandy RA, Montecino M, van Wijnen AJ, Lian JB, Stein GS, Stein JL. Tai PW, et al. J Cell Physiol. 2014 Jun;229(6):711-27. doi: 10.1002/jcp.24508. J Cell Physiol. 2014. PMID: 24242872 Free PMC article. Review. - The nuclear basket proteins Mlp1p and Mlp2p are part of a dynamic interactome including Esc1p and the proteasome.
Niepel M, Molloy KR, Williams R, Farr JC, Meinema AC, Vecchietti N, Cristea IM, Chait BT, Rout MP, Strambio-De-Castillia C. Niepel M, et al. Mol Biol Cell. 2013 Dec;24(24):3920-38. doi: 10.1091/mbc.E13-07-0412. Epub 2013 Oct 23. Mol Biol Cell. 2013. PMID: 24152732 Free PMC article. - Sumoylation and transcription regulation at nuclear pores.
Texari L, Stutz F. Texari L, et al. Chromosoma. 2015 Mar;124(1):45-56. doi: 10.1007/s00412-014-0481-x. Epub 2014 Aug 30. Chromosoma. 2015. PMID: 25171917 Free PMC article. Review.
References
- Cabal GG, Genovesio A, Rodriguez-Navarro S, Zimmer C, Gadal O, Lesne A, Buc H, Feuerbach-Fournier F, Olivo-Marin JC, Hurt EC, et al. SAGA interacting factors confine sub-diffusion of transcribed genes to the nuclear envelope. Nature. 2006;441:770–773. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous