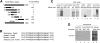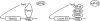Pumilio 2 controls translation by competing with eIF4E for 7-methyl guanosine cap recognition - PubMed (original) (raw)
Pumilio 2 controls translation by competing with eIF4E for 7-methyl guanosine cap recognition
Quiping Cao et al. RNA. 2010 Jan.
Abstract
Pumilio 2 (Pum2) interacts with the 3' UTR-containing pumilio binding element (PBE) of RINGO/SPY mRNA to repress translation in Xenopus oocytes. Here, we show that Pum2 also binds directly to the 5' 7mG cap structure; in so doing, it precludes eIF4E from binding the cap. Using deletion analysis, we have mapped the cap interaction domain of Pum2 to the amino terminus of the protein and identified a conserved tryptophan residue that mediates this specific interaction. Reporter mRNA-based assays demonstrate that Pum2 requires the conserved tryptophan to repress translation in injected Xenopus oocytes. Thus, in addition to its suggested role in regulating poly(A) tail length and mRNA stability, our results suggest that vertebrate Pumilio can repress translation by blocking the assembly of the essential initiation complex on the cap.
Figures
FIGURE 1.
Pum2 binds the cap. (A) Cytostatic Factor (CSF) extracts were prepared from Xenopus eggs and subsequently supplemented with calcium to induce entry into the cell cycle. At 0, 10, and 20 min after calcium addition, the extracts were supplemented with GTP and applied to m7G-Sepharose resin (lanes 1–3). In addition, some extract (no calcium) was supplemented with cap analog and also applied to the cap analog resin (lane 7). The proteins that were retained on the m7G-Sepharose resin were probed on Western blots for Maskin, Pum2, and eIF4E. The load fractions (i.e., 10% of total initial extract) were also probed for the same proteins (lanes 4–6). (B) Xenopus oocyte extracts were applied to GDP-Sepharose or m7G-Sepharose; the bound material was probed for Pum2 and eIF4E on a Western blot. The load fraction (10% of total) was also probed for the same proteins. (C) Reticulocyte lysates were primed with mRNA encoding Pum2 or eIF4E in the presence of 35S-methionine (lanes 3,4). Equal volumes of the lysates were mixed, supplemented with GTP or cap analog, and applied to m7G-Sepharose. Pum2 and eIF4E that were retained on the resin were detected by SDS-PAGE and phosphorimaging. (D) E. coli.-expressed Pum2 and eIF4E were applied to m7G-Sepharose or GDP-Sepharose columns; the bound material was examined by Western blotting (lanes 1–4). Ten percent of the load fractions were also probed by Western blotting (lanes 5,6). (E) Varying amounts of reticulocyte-synthesized Pum2 and eIF4E (i.e., μL of lysate) were applied to m7G-Sepharose resin in the presence of GTP or GTP plus cap analog and the amount retained was analyzed by Western blotting (top, bottom). In some cases, the lysates were mixed in the amounts indicated prior to being applied to m7G-Sepharose (bottom panel, lanes 1–5). In these cases, the lysates contained GTP but no free cap analog.
FIGURE 2.
Pum2 W344 mediates cap binding and translational repression. (A) Pum2 deletion mutants and a W344G point mutant were expressed in reticulocyte lysates and applied to m7G-Sepharose; the percent bound was determined by Western blotting. (B) Sequence alignment of a selected region of Pum2 protein among animal species. The underlined W (corresponding to W344 in Xenopus laevis) is conserved among these and probably most other vertebrates as well. (C) Pum2 WT, Pum2 W344G, eIF4E, and ePAB were expressed in reticulocyte lysates, applied to GDP-Sepharose or m7G-Sepharose, and proteins that were retained were analyzed by Western blotting. (D) Recombinant Pum2 WT and W344G were applied to m7G-Sepharose and the bound material analyzed by Western blotting.
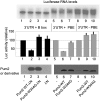
FIGURE 3.
Pum2 represses translation in a W344-dependent manner. RNAs encoding Pum2 WT and W344G fused to λ N protein were injected into 10 oocytes. Following an overnight incubation, the oocytes were then injected with luciferase RNA containing 5 B boxes in the 3′ UTR. The oocytes were then homogenized and luciferase activity was determined (histogram) as was expression of the fusion proteins by Western blotting (bottom). The top portion shows an autoradiogram indicating that the relative levels of 32P-UTP trace-labeled luciferase RNAs at the end of the incubation period were similar. Other RNAs encoding Pum2 WT and W344G (no fusion) were injected into oocytes with luciferase RNA, whose 3′ UTR contained or lacked a PBE. Luciferase activity, expression of the heterologous proteins, and relative amount of the luciferase reporter RNAs that remained after the incubation period were determined as above. The amount of luciferase activity in the absence of heterologous Pum2 was used as the standard against which the other values were normalized. Each experiment was performed three times; the bars on the histograms refer to SEM. The data are statistically significant (P < 0.05, Student's _t_-test).
FIGURE 4.
Pum2 and CPEB-mediated translational control. Comparison of the Pum2–cap interaction with the CPEB–Maskin–eIF4E interaction. The PBE refers to the pumilio binding element and the CPE to the cytoplasmic polyadenylation element.
Similar articles
- Regulated Pumilio-2 binding controls RINGO/Spy mRNA translation and CPEB activation.
Padmanabhan K, Richter JD. Padmanabhan K, et al. Genes Dev. 2006 Jan 15;20(2):199-209. doi: 10.1101/gad.1383106. Genes Dev. 2006. PMID: 16418484 Free PMC article. - CPEB interacts with an ovary-specific eIF4E and 4E-T in early Xenopus oocytes.
Minshall N, Reiter MH, Weil D, Standart N. Minshall N, et al. J Biol Chem. 2007 Dec 28;282(52):37389-401. doi: 10.1074/jbc.M704629200. Epub 2007 Oct 17. J Biol Chem. 2007. PMID: 17942399 - eIF4E3 acts as a tumor suppressor by utilizing an atypical mode of methyl-7-guanosine cap recognition.
Osborne MJ, Volpon L, Kornblatt JA, Culjkovic-Kraljacic B, Baguet A, Borden KL. Osborne MJ, et al. Proc Natl Acad Sci U S A. 2013 Mar 5;110(10):3877-82. doi: 10.1073/pnas.1216862110. Epub 2013 Feb 19. Proc Natl Acad Sci U S A. 2013. PMID: 23431134 Free PMC article. - Cap-dependent translation initiation factor eIF4E: an emerging anticancer drug target.
Jia Y, Polunovsky V, Bitterman PB, Wagner CR. Jia Y, et al. Med Res Rev. 2012 Jul;32(4):786-814. doi: 10.1002/med.21260. Epub 2012 Apr 11. Med Res Rev. 2012. PMID: 22495651 Free PMC article. Review. - [Translational control by the poly(A) binding protein: a check for mRNA integrity].
Svitkin YV, Sonenberg N. Svitkin YV, et al. Mol Biol (Mosk). 2006 Jul-Aug;40(4):684-93. Mol Biol (Mosk). 2006. PMID: 16913227 Review. Russian.
Cited by
- Autoregulation of Musashi1 mRNA translation during Xenopus oocyte maturation.
Arumugam K, Macnicol MC, Macnicol AM. Arumugam K, et al. Mol Reprod Dev. 2012 Aug;79(8):553-63. doi: 10.1002/mrd.22060. Epub 2012 Jul 9. Mol Reprod Dev. 2012. PMID: 22730340 Free PMC article. - The retrotransposon-derived capsid genes PNMA1 and PNMA4 maintain reproductive capacity.
Wood TWP, Henriques WS, Cullen HB, Romero M, Blengini CS, Sarathy S, Sorkin J, Bekele H, Jin C, Kim S, Wang X, Laureau R, Chemiakine A, Khondker RC, Isola JVV, Stout MB, Gennarino VA, Mogessie B, Jain D, Schindler K, Suh Y, Wiedenheft B, Berchowitz LE. Wood TWP, et al. Nat Aging. 2025 May;5(5):765-779. doi: 10.1038/s43587-025-00852-y. Epub 2025 Apr 22. Nat Aging. 2025. PMID: 40263616 - Rhox13 is translated in premeiotic germ cells in male and female mice and is regulated by NANOS2 in the male.
Geyer CB, Saba R, Kato Y, Anderson AJ, Chappell VK, Saga Y, Eddy EM. Geyer CB, et al. Biol Reprod. 2012 Apr 27;86(4):127. doi: 10.1095/biolreprod.111.094938. Print 2012 Apr. Biol Reprod. 2012. PMID: 22190708 Free PMC article. - Alternative Polyadenylation in Triple-Negative Breast Tumors Allows NRAS and c-JUN to Bypass PUMILIO Posttranscriptional Regulation.
Miles WO, Lembo A, Volorio A, Brachtel E, Tian B, Sgroi D, Provero P, Dyson N. Miles WO, et al. Cancer Res. 2016 Dec 15;76(24):7231-7241. doi: 10.1158/0008-5472.CAN-16-0844. Epub 2016 Oct 10. Cancer Res. 2016. PMID: 27758885 Free PMC article. - Functions, mechanisms and regulation of Pumilio/Puf family RNA binding proteins: a comprehensive review.
Nishanth MJ, Simon B. Nishanth MJ, et al. Mol Biol Rep. 2020 Jan;47(1):785-807. doi: 10.1007/s11033-019-05142-6. Epub 2019 Oct 23. Mol Biol Rep. 2020. PMID: 31643042 Review.
References
- Barnard DC, Ryan K, Manley JL, Richter JD. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell. 2004;119:641–645. - PubMed
- Baron-Benhamou J, Gehring NH, Kulozik AE, Hentze MW. Using the λN peptide to tether proteins to RNAs. Methods Mol Biol. 2004;257:135–154. - PubMed
- Cao Q, Kim JH, Richter JD. CDK1 and calcineurin regulate Maskin association with eIF4E and translational control of cell cycle progression. Nat Struct Mol Biol. 2006;13:1128–1133. - PubMed
- Cho PF, Poulin F, Cho-Park YA, Cho-Park IB, Chicoine JD, Lasko P, Sonenberg N. A new paradigm for translational control: Inhibition via 5′–3′ mRNA tethering by Bicoid and the eIF4E cognate 4EH. Cell. 2005;121:411–423. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous