Carboplatin and Paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer - PubMed (original) (raw)
Clinical Trial
Carboplatin and Paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer
Suresh S Ramalingam et al. J Clin Oncol. 2010.
Abstract
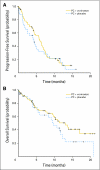
PURPOSE Vorinostat, a histone deacetylase inhibitor, exerts anticancer effects by both histone and nonhistone-mediated mechanisms. It also enhances the anticancer effects of platinum compounds and taxanes in non-small-cell lung cancer (NSCLC) cell lines. This phase II randomized, double-blinded, placebo-controlled study evaluated the efficacy of vorinostat in combination with carboplatin and paclitaxel in patients with advanced-stage NSCLC. PATIENTS AND METHODS Patients with previously untreated stage IIIB (ie, wet) or IV NSCLC were randomly assigned (2:1) to carboplatin (area under the curve, 6 mg/mL x min) and paclitaxel (200 mg/m(2) day 3) with either vorinostat (400 mg by mouth daily) or placebo. Vorinostat or placebo was given on days 1 through 14 of each 3-week cycle to a maximum of six cycles. The primary end point was comparison of the response rate. Results Ninety-four patients initiated protocol therapy. Baseline patient characteristics were similar between the two arms. The median number of cycles was four for both treatment arms. The confirmed response rate was 34% with vorinostat versus 12.5% with placebo (P = .02). There was a trend toward improvement in median progression-free survival (6.0 months v 4.1 months; P = .48) and overall survival (13.0 months v 9.7 months; P = .17) in the vorinostat arm. Grade 4 platelet toxicity was more common with vorinostat (18% v 3%; P < .05). Nausea, emesis, fatigue, dehydration, and hyponatremia also were more frequent with vorinostat. CONCLUSION Vorinostat enhances the efficacy of carboplatin and paclitaxel in patients with advanced NSCLC. HDAC inhibition is a promising therapeutic strategy for treatment of NSCLC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
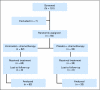
Fig 1.
CONSORT diagram.
Fig 2.
(A) Kaplan-Meier curve for progression-free survival. (B) Kaplan-Meier curve for overall survival. PC, paclitaxel and carboplatin.
Similar articles
- Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study.
Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D, Zukin M, Walde D, Laberge F, Vincent MD, Ellis PM, Laurie SA, Ding K, Frymire E, Gauthier I, Leighl NB, Ho C, Noble J, Lee CW, Seymour L. Goss GD, et al. J Clin Oncol. 2010 Jan 1;28(1):49-55. doi: 10.1200/JCO.2009.22.9427. Epub 2009 Nov 16. J Clin Oncol. 2010. PMID: 19917841 Clinical Trial. - Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer.
Lara PN Jr, Douillard JY, Nakagawa K, von Pawel J, McKeage MJ, Albert I, Losonczy G, Reck M, Heo DS, Fan X, Fandi A, Scagliotti G. Lara PN Jr, et al. J Clin Oncol. 2011 Aug 1;29(22):2965-71. doi: 10.1200/JCO.2011.35.0660. Epub 2011 Jun 27. J Clin Oncol. 2011. PMID: 21709202 Clinical Trial. - A randomized, double-blind, placebo-controlled phase 2 study of tigatuzumab (CS-1008) in combination with carboplatin/paclitaxel in patients with chemotherapy-naïve metastatic/unresectable non-small cell lung cancer.
Reck M, Krzakowski M, Chmielowska E, Sebastian M, Hadler D, Fox T, Wang Q, Greenberg J, Beckman RA, von Pawel J. Reck M, et al. Lung Cancer. 2013 Dec;82(3):441-8. doi: 10.1016/j.lungcan.2013.09.014. Epub 2013 Oct 1. Lung Cancer. 2013. PMID: 24148258 Clinical Trial. - Randomized phase II study of carboplatin and paclitaxel with either linifanib or placebo for advanced nonsquamous non-small-cell lung cancer.
Ramalingam SS, Shtivelband M, Soo RA, Barrios CH, Makhson A, Segalla JG, Pittman KB, Kolman P, Pereira JR, Srkalovic G, Belani CP, Axelrod R, Owonikoko TK, Qin Q, Qian J, McKeegan EM, Devanarayan V, McKee MD, Ricker JL, Carlson DM, Gorbunova VA. Ramalingam SS, et al. J Clin Oncol. 2015 Feb 10;33(5):433-41. doi: 10.1200/JCO.2014.55.7173. Epub 2015 Jan 5. J Clin Oncol. 2015. PMID: 25559798 Free PMC article. Clinical Trial. - Phase II study of carboplatin-paclitaxel combination chemotherapy in elderly patients with advanced non-small cell lung cancer.
Okamoto I, Moriyama E, Fujii S, Kishi H, Nomura M, Goto E, Kiyofuji C, Imamura F, Mori T, Matsumoto M. Okamoto I, et al. Jpn J Clin Oncol. 2005 Apr;35(4):188-94. doi: 10.1093/jjco/hyi059. Jpn J Clin Oncol. 2005. PMID: 15845567 Review.
Cited by
- Emerging new agents for the management of patients with non-small cell lung cancer.
Capelletto E, Novello S. Capelletto E, et al. Drugs. 2012 Jun 19;72 Suppl 1:37-52. doi: 10.2165/1163028-S0-000000000-00000. Drugs. 2012. PMID: 22712796 Review. - Epigenetic therapy: use of agents targeting deacetylation and methylation in cancer management.
Ho AS, Turcan S, Chan TA. Ho AS, et al. Onco Targets Ther. 2013;6:223-32. doi: 10.2147/OTT.S34680. Epub 2013 Mar 21. Onco Targets Ther. 2013. PMID: 23569385 Free PMC article. - Role of Hydroxamate-Based Histone Deacetylase Inhibitors (Hb-HDACIs) in the Treatment of Solid Malignancies.
Grassadonia A, Cioffi P, Simiele F, Iezzi L, Zilli M, Natoli C. Grassadonia A, et al. Cancers (Basel). 2013 Jul 25;5(3):919-42. doi: 10.3390/cancers5030919. Cancers (Basel). 2013. PMID: 24202327 Free PMC article. - Epigenetic Therapeutics: A New Weapon in the War Against Cancer.
Ahuja N, Sharma AR, Baylin SB. Ahuja N, et al. Annu Rev Med. 2016;67:73-89. doi: 10.1146/annurev-med-111314-035900. Annu Rev Med. 2016. PMID: 26768237 Free PMC article. Review. - Hypoxia in Lung Cancer Management: A Translational Approach.
Ancel J, Perotin JM, Dewolf M, Launois C, Mulette P, Nawrocki-Raby B, Dalstein V, Gilles C, Deslée G, Polette M, Dormoy V. Ancel J, et al. Cancers (Basel). 2021 Jul 8;13(14):3421. doi: 10.3390/cancers13143421. Cancers (Basel). 2021. PMID: 34298636 Free PMC article. Review.
References
- Ramalingam S, Belani CP. State-of-the-art chemotherapy for advanced non–small-cell lung cancer. Semin Oncol. 2004;31(suppl 1):68–74. - PubMed
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non–small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. - PubMed
- Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non–small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. - PubMed
- Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non–small-cell lung cancer (FLEX): An open-label randomised phase III trial. Lancet. 2009;373:1525–1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- N01CM62208/CM/NCI NIH HHS/United States
- N01-CM-62208/CM/NCI NIH HHS/United States
- N01CM62209/CA/NCI NIH HHS/United States
- N01-CM-62201/CM/NCI NIH HHS/United States
- CM-62209/CM/NCI NIH HHS/United States
- UL1 TR000005/TR/NCATS NIH HHS/United States
- N01CM62201/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical