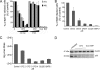Dynamin- and clathrin-dependent endocytosis in African swine fever virus entry - PubMed (original) (raw)
Dynamin- and clathrin-dependent endocytosis in African swine fever virus entry
Bruno Hernaez et al. J Virol. 2010 Feb.
Abstract
African swine fever virus (ASFV) is a large DNA virus that enters host cells after receptor-mediated endocytosis and depends on acidic cellular compartments for productive infection. The exact cellular mechanism, however, is largely unknown. In order to dissect ASFV entry, we have analyzed the major endocytic routes using specific inhibitors and dominant negative mutants and analyzed the consequences for ASFV entry into host cells. Our results indicate that ASFV entry into host cells takes place by clathrin-mediated endocytosis which requires dynamin GTPase activity. Also, the clathrin-coated pit component Eps15 was identified as a relevant cellular factor during infection. The presence of cholesterol in cellular membranes, but not lipid rafts or caveolae, was found to be essential for a productive ASFV infection. In contrast, inhibitors of the Na(+)/H(+) ion channels and actin polymerization inhibition did not significantly modify ASFV infection, suggesting that macropinocytosis does not represent the main entry route for ASFV. These results suggest a dynamin-dependent and clathrin-mediated endocytic pathway of ASFV entry for the cell types and viral strains analyzed.
Figures
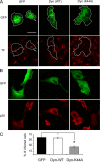
FIG. 1.
Inhibition of ASFV infection by transient expression of dynamin dominant negative mutant K44A. Vero cells were transfected with GFP, wild-type dynamin-GFP, and K44A-GFP plasmids. (A) At 24 h after transfection, cells were examined by confocal microscopy for TF-A594 uptake. Bar, 30 μm. (B) Likewise, transfected cells were infected with BA71V, and infected cells at 6 hpi were detected as those positive for immunofluorescence with anti-p30 monoclonal antibody followed by anti-mouse Alexa 594-conjugated secondary antibody. (C) Percentages of transfected and infected cells. More than 100 transfected cells were examined in each case, and the means and standard deviations correspond to three independent experiments (asterisk, P < 0.05).
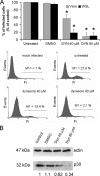
FIG. 2.
ASFV infection is sensitive to dynamin inhibition by dynasore. (A) Vero and WSL cells were pretreated with different concentrations of dynasore and then infected with BA71V for 6 h. Infected cells were detected by FACS and data normalized to infection rates in untreated cells. Error bars indicate standard deviations from three independent experiments. Representative FACS profiles (events versus fluorescence intensity [Fi]) obtained during the analysis are shown below the graphs. Infected cells were gated in M1 and expressed as a percentage of total cell analyzed. (B) As previously, Vero cells were incubated with dynasore and then infected with BA71V. Cells were lysed at 6 hpi, and p30 expression was monitored by Western blotting. β-Actin was detected as control for protein loading. Results are from a representative experiment of three independent experiments performed. Quantification of the bands corresponding to p30 was corrected with β-actin data and then normalized to control values.
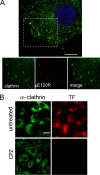
FIG. 3.
Colocalization of ASFV with clathrin by confocal microscopy. (A) Vero cells infected with BA71V (>10 PFU/cell) and fixed at 0 to 20 min after adsorption were incubated with anti-ASFV structural protein pE120R (red) and anti-clathrin heavy chain (green) antibodies, followed by anti-rabbit IgG and anti-mouse IgG conjugated to A594 and A488, respectively. Colocalization of virions with clathrin can be observed in the representative 0.1-μm optical section at 5 min postinfection with nuclear staining TOPRO3 (blue). Bar, 15 μm. (B) Chlorpromazine inhibition of endocytosis. Vero cells were examined by confocal microscopy for TF-A594 uptake after treatment with different chlorpromazine (CPZ) concentrations. Only chlorpromazine at 14 μM is shown. The clathrin distribution was analyzed with a specific monoclonal antibody followed by anti-mouse conjugated to Alexa 488. Bar, 20 μm.
FIG. 4.
CPZ inhibition of ASFV infection. (A and B) After CPZ treatment, Vero and WSL cells were infected with strain BA71V (A) or 608 VR13 (B), and infected cells were detected by FACS at 6 hpi, after staining with monoclonal anti-p30 antibody. The drug was added 30 min before infection or after 3 h of infection when indicated. Percentages relative to total infected cells found in controls are shown. The lysosomotropic agents chloroquine (CLQ) and bafilomycin A1 (BAF) were included as controls of infection inhibition. Error bars indicate standard deviations. (C) Effect of chlorpromazine on ASFV yields. Vero cells were incubated with different concentrations of chlorpromazine (CPZ) and then infected with BA71V (MOI, <1 PFU/cell). At 36 hpi, cells and media were harvested and virus titers analyzed by plaque assay (see Materials and Methods). (D) Inhibition of viral protein synthesis was analyzed by Western blotting of infected cells extracts with monoclonal anti-ASFV protein p30 at 6 hpi. Increasing concentrations of CPZ were used, and β-actin was detected as control of protein loading. In both cases, chloroquine (CLQ) and bafilomycin A1 (BAF) were included as controls.
FIG. 5.
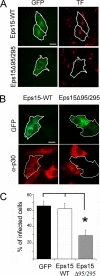
Inhibition of ASFV entry by dominant negative mutant protein Eps15. Vero cells were transfected with plasmids encoding either wild-type Eps15-GFP or dominant negative mutant Eps15Δ95/295-GFP. (A) Transfected cells were examined by confocal microscopy for TF-A594 uptake to test the effectiveness of Eps15Δ95/295 for inhibition of clathrin-mediated endocytosis. (B) At 24 h after transfection, cells were infected with BA71V (MOI = 1 PFU/cell), and infected cells were detected at 6 hpi by immunofluorescence using anti-p30 monoclonal antibody followed by anti-mouse conjugated to Alexa 594 as a secondary antibody. Bar, 20 μm. (C) The numbers of transfected and simultaneously infected cells are expressed as percentages of viral antigen-positive cells. The means and standard deviations shown correspond to three experiments (>100 cells in each case).
FIG. 6.
Cholesterol is required during early stages of ASFV infection. (A) Internalization of cholera toxin (CTX) conjugated to Alexa 488 was examined by confocal microscopy in Vero cells previously treated with methyl-β-cyclodextrin (CD) and nystatin (NYS). Bar, 20 μm. (B) Cholesterol levels in cell extracts were determined after treatment with the different drugs used in the study. (C) The effects of cholesterol reorganization (NYS) and cholesterol depletion (CD) on ASFV infection are shown as percentages of infected cells at 6 hpi determined by FACS, normalized to total numbers of infected cells in untreated cultures. Infections with the BA71V isolate in Vero and WSL cells were compared after CD and NYS treatments (upper graph). Likewise, infection with 608VR13 isolate in Vero cells was analyzed after CD and NYS treatments (lower graph). The means and standard deviations shown correspond to three independent experiments. (D) Effects of NYS and CD on viral protein synthesis at 6 hpi were determined by Western blotting with specific antibodies.
FIG. 7.
Effect of ion channel blockers and actin polymerization inhibitors on ASFV infection. (A) To monitor the effectiveness of ion channel inhibitor EIPA, Vero cells were treated with different concentrations and examined for dextran-Alexa 594 uptake using confocal microscopy. Internalized dextran in control cells can be seen as a punctate staining close to the nucleus. Bar, 20 μm. Quantification of internalized dextran after EIPA treatment is also shown. (B) The number of infected cells at 6 hpi was scored by FACS after EIPA, jasplakinolide, and latrunculin A treatment. Error bars indicate standard deviations. (C) Cells were infected after EIPA treatment, and expression of early viral protein p30 was determined by Western blotting at 6 hpi.
Similar articles
- African swine fever virus infects macrophages, the natural host cells, via clathrin- and cholesterol-dependent endocytosis.
Galindo I, Cuesta-Geijo MA, Hlavova K, Muñoz-Moreno R, Barrado-Gil L, Dominguez J, Alonso C. Galindo I, et al. Virus Res. 2015 Mar 16;200:45-55. doi: 10.1016/j.virusres.2015.01.022. Epub 2015 Feb 3. Virus Res. 2015. PMID: 25662020 - Clathrin- and caveolae-independent entry of feline infectious peritonitis virus in monocytes depends on dynamin.
Van Hamme E, Dewerchin HL, Cornelissen E, Verhasselt B, Nauwynck HJ. Van Hamme E, et al. J Gen Virol. 2008 Sep;89(Pt 9):2147-2156. doi: 10.1099/vir.0.2008/001602-0. J Gen Virol. 2008. PMID: 18753224 - Entry of Classical Swine Fever Virus into PK-15 Cells via a pH-, Dynamin-, and Cholesterol-Dependent, Clathrin-Mediated Endocytic Pathway That Requires Rab5 and Rab7.
Shi BJ, Liu CC, Zhou J, Wang SQ, Gao ZC, Zhang XM, Zhou B, Chen PY. Shi BJ, et al. J Virol. 2016 Sep 29;90(20):9194-208. doi: 10.1128/JVI.00688-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489278 Free PMC article. - Spatiotemporally Orchestrated Interactions between Viral and Cellular Proteins Involved in the Entry of African Swine Fever Virus.
Zhang K, Li S, Liu S, Li S, Qu L, Gao GF, Qiu HJ. Zhang K, et al. Viruses. 2021 Dec 13;13(12):2495. doi: 10.3390/v13122495. Viruses. 2021. PMID: 34960765 Free PMC article. Review. - African swine fever virus-cell interactions: from virus entry to cell survival.
Alonso C, Galindo I, Cuesta-Geijo MA, Cabezas M, Hernaez B, Muñoz-Moreno R. Alonso C, et al. Virus Res. 2013 Apr;173(1):42-57. doi: 10.1016/j.virusres.2012.12.006. Epub 2012 Dec 20. Virus Res. 2013. PMID: 23262167 Free PMC article. Review.
Cited by
- Modulation of Host Antiviral Innate Immunity by African Swine Fever Virus: A Review.
He WR, Yuan J, Ma YH, Zhao CY, Yang ZY, Zhang Y, Han S, Wan B, Zhang GP. He WR, et al. Animals (Basel). 2022 Oct 26;12(21):2935. doi: 10.3390/ani12212935. Animals (Basel). 2022. PMID: 36359059 Free PMC article. Review. - African Swine Fever Virus Ubiquitin-Conjugating Enzyme Interacts With Host Translation Machinery to Regulate the Host Protein Synthesis.
Barrado-Gil L, Del Puerto A, Muñoz-Moreno R, Galindo I, Cuesta-Geijo MÁ, Urquiza J, Nistal-Villán E, Maluquer de Motes C, Alonso C. Barrado-Gil L, et al. Front Microbiol. 2020 Dec 15;11:622907. doi: 10.3389/fmicb.2020.622907. eCollection 2020. Front Microbiol. 2020. PMID: 33384682 Free PMC article. - Small rho GTPases and cholesterol biosynthetic pathway intermediates in African swine fever virus infection.
Quetglas JI, Hernáez B, Galindo I, Muñoz-Moreno R, Cuesta-Geijo MA, Alonso C. Quetglas JI, et al. J Virol. 2012 Feb;86(3):1758-67. doi: 10.1128/JVI.05666-11. Epub 2011 Nov 23. J Virol. 2012. PMID: 22114329 Free PMC article. - Recent progress and major gaps in the vaccine development for African swine fever.
Chandana MS, Nair SS, Chaturvedi VK, Abhishek, Pal S, Charan MSS, Balaji S, Saini S, Vasavi K, Deepa P. Chandana MS, et al. Braz J Microbiol. 2024 Mar;55(1):997-1010. doi: 10.1007/s42770-024-01264-7. Epub 2024 Feb 5. Braz J Microbiol. 2024. PMID: 38311710 Review. - Small peptide inhibitors disrupt a high-affinity interaction between cytoplasmic dynein and a viral cargo protein.
Hernáez B, Tarragó T, Giralt E, Escribano JM, Alonso C. Hernáez B, et al. J Virol. 2010 Oct;84(20):10792-801. doi: 10.1128/JVI.01168-10. Epub 2010 Aug 4. J Virol. 2010. PMID: 20686048 Free PMC article.
References
- Alcami, A., A. L. Carrascosa, and E. Vinuela. 1989. The entry of African swine fever virus into Vero cells. Virology 171:68-75. - PubMed
- Alfonso, P., J. Rivera, B. Hernaez, C. Alonso, and J. M. Escribano. 2004. Identification of cellular proteins modified in response to African swine fever virus infection by proteomics. Proteomics 4:2037-2046. - PubMed
- Alonso, C., J. Miskin, B. Hernaez, P. Fernandez-Zapatero, L. Soto, C. Canto, I. Rodriguez-Crespo, L. Dixon, and J. M. Escribano. 2001. African swine fever virus protein p54 interacts with the microtubular motor complex through direct binding to light-chain dynein. J. Virol. 75:9819-9827. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous