VAMP8/endobrevin is overexpressed in hyperreactive human platelets: suggested role for platelet microRNA - PubMed (original) (raw)
VAMP8/endobrevin is overexpressed in hyperreactive human platelets: suggested role for platelet microRNA
A A Kondkar et al. J Thromb Haemost. 2010 Feb.
Abstract
Background: Variation in platelet reactivity contributes to disorders of hemostasis and thrombosis, but the molecular mechanisms are not well understood.
Objectives: To discover associations between interindividual platelet variability and the responsible platelet genes, and to begin to define the molecular mechanisms altering platelet gene expression.
Subjects/methods: Two hundred and eighty-eight healthy subjects were phenotyped for platelet responsiveness. Platelet RNA from subjects demonstrating hyperreactivity (n=18) and hyporeactivity (n=11) was used to screen the human transcriptome.
Results: Distinctly different mRNA profiles were observed between subjects with differing platelet reactivity. Increased levels of mRNA for VAMP8/endobrevin, a critical v-SNARE involved in platelet granule secretion, were associated with platelet hyperreactivity (Q=0.0275). Validation studies of microarray results showed 4.8-fold higher mean VAMP8 mRNA levels in hyperreactive than hyporeactive platelets (P=0.0023). VAMP8 protein levels varied 13-fold among platelets from these normal subjects, and were 2.5-fold higher in hyperreactive platelets (P=0.05). Among our cohort of 288 subjects, a VAMP8 single-nucleotide polymorphism (rs1010) was associated with platelet reactivity in an age-dependent manner (P<0.003). MicroRNA-96 was predicted to bind to the 3'-untranslated regionof VAMP8 mRNA and was detected in platelets. Overexpression of microRNA-96 in VAMP8-expressing cell lines caused a dose-dependent decrease in VAMP8 protein and mRNA, suggesting a role in VAMP8 mRNA degradation.
Conclusions: These findings support a role for VAMP8/endobrevin in the heterogeneity of platelet reactivity, and suggest a role for microRNA-96 in the regulation of VAMP8 expression.
Conflict of interest statement
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Figures
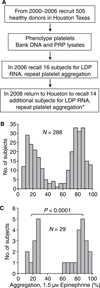
Fig. 1
Distribution of platelet aggregation response in study subjects. (A) Strategy for subject recruitment. Note that preparation of leukocyte-depleted platelets (LDPs) was not part of the original protocol. *One subject was excluded because epinephrine-induced platelet reactivity was inconsistent over time. (B, C) Histograms depicting the number of subjects (_y_-axis) with a given level of platelet aggregation (_x_-axis) in response to 1.5 µ
m
epinephrine (B) in the whole cohort and (C) in the subjects available for participation in the current study. PRP, platelet-rich plasma.
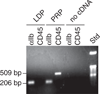
Fig. 2
Assessment of leukocyte depleted-platelet (LDP) RNA purity. Ethidium bromide-stained agarose gel containing reverse transcription polymerase chain reaction-amplified products of mRNA for platelet integrin αIIb (a platelet-specific marker) and CD45 (the ‘common leukocyte antigen,’ a leukocyte marker) after 30 cycles of polymerase chain reaction. PRP, platelet-rich plasma; Std, standard.
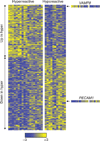
Fig. 3
Differential expression of 290 transcripts in platelets of differing reactivity. The heatmap represents the normalized data values for the genes (each row) identified as being significantly differentially expressed between leukocyte-depleted platelet hyperreactive and hyporeactive samples. The genes were ordered by the mean difference in variance stabilizing transformation expression values between hyperreactive and hyporeactive samples. The order of the samples within each group was determined according to the mean rank of the centered and scaled expression values for the 122 upregulated transcripts and the 168 downregulated transcripts within each respective group. The VAMP8 and PECAM1 heatmaps are expanded for more clear visualization. PECAM1 was selected as a well-characterized platelet gene. Notably, its expression was reduced in the hyperreactive platelets, consistent with the known inhibitory function of PECAM1 in platelets.
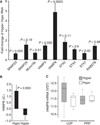
Fig. 4
Relative quantification of secretory genes. (A) mRNA fold-changes of hyperreactive platelets as compared with hyporeactive platelets. mRNA levels were analyzed by TaqMan assay on the leukocyte-depleted platelet (LDP) amplified RNA (aRNA) isolated from hyperreactive (n = 10) and hyporeactive (n = 6) platelets. aRNA (300 ng) was reverse transcribed, and 15 ng of cDNA was subjected to real-time polymerase chain reaction (PCR). (B) Instead of fold-change, this figure shows the normalized real-time PCR cycle threshold values (Δ_C_T) for VAMP8 (_y_-axis) in the hyperreactive and hyporeactive subjects (_x_-axis) in (A). Δ_C_T represents the real-time PCR cycle threshold, CT, value for target that has been normalized by subtracting the control _C_T value [i.e. Δ_C_T = _C_T (target gene) − _C_T (control gene)]. The glyceraldehyde-3-phosphate dehydrogenase gene (GAPDH) was used as control. (C) Box plot showing normalized RNA [variance stabilizing transformation (VST)] levels for VAMP8 from LDP and platelet-rich plasma (PRP) RNA preparations. RNA expression was also performed using PRP from 11 (six hypers and five hypos) of the 29 recalled subjects.
Fig. 5
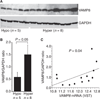
Differential expression of VAMP8 protein. (A) Twenty micrograms of platelet lysates from platelet-rich plasma were separated by 15% sodium dodecylsulfate polyacrylamide gel electrophoresis, and the same filter was immunoblotted for VAMP8 and glyceraldehyde-3-phosphate dehydrogenase (GADPH). (B) Relative quantification was performed using an LI-COR Odyssey image analyzer. (C) Correlation between VAMP8 protein and mRNA. VAMP8 mRNA levels represent the variance stabilizing transformation (VST) normalized microarray values (r = 0.5).
Fig. 6
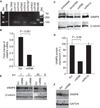
Effect of miR-96 on VAMP8 expression. (A) Total leukocyte-depleted platelet RNA (20 ng) was reverse transcribed, and 7 ng of the resulting cDNA was used for real-time quantification, using TaqMan for each indicated miRNA. Small nuclear RNA, RNU6B, was used as a positive control. (B) Fold-change in VAMP8 mRNA levels in HCT116-Dicer-KO cells transfected with 40 pmol of miR-96 at 48 h as compared with cells transfected with a scrambled miR (three independent experiments). (C) Immunoblot of VAMP8 protein in HCT116-Dicer-KO cells transfected with 40 pmol of indicated miRNAs for 48 h. All lanes are from the same gel, but an intervening lane between miR96 and miR410 was deleted. (D) The mean normalized VAMP8 protein levels at 48 h after miRNA transfection in three (miR-96) or two (miR-410) independent experiments. Data are shown as percentage expression as compared with cells transfected with a scrambled miR. VAMP8 levels were normalized to β-tubulin. (E) Dose–response of VAMP8 protein after HCT116-Dicer-KO cells were transfected with increasing concentrations of miR-96 (in pmoles). (F) Immunoblot of VAMP8 protein in HEL cells transfected with the indicated miRNAs for 48 h. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Scr, scrambled miRNA control; U, untransfected HCT116-Dicer–KO cells; Std, standard.
Fig. 7
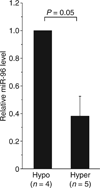
MiRNA-96 expression levels in subjects with differing platelet reactivity.MiR-96 levels were quantified using TaqMan polymerase chain reaction, and normalized to glyceraldehyde-3-phosphate dehydrogenase, as described in Fig. 4B. Δ_C_T data have been converted to relative levels of miR-96 in hyperreactive (n = 5) platelets as compared with hyporeactive (n = 4) platelets.
Similar articles
- No influence of the VAMP8 rs1010 single nucleotide polymorphism on platelet functions in vitro.
Gaussem P, Ishida BY, Fontana P, Pullinger CR, Khane JP, Aiach M, Bachelot-Loza C, Gandrille S. Gaussem P, et al. J Cell Mol Med. 2009 Mar;13(3):601-3. doi: 10.1111/j.1582-4934.2009.00500_2.x. J Cell Mol Med. 2009. PMID: 19374688 Free PMC article. No abstract available. - Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity.
Nagalla S, Shaw C, Kong X, Kondkar AA, Edelstein LC, Ma L, Chen J, McKnight GS, López JA, Yang L, Jin Y, Bray MS, Leal SM, Dong JF, Bray PF. Nagalla S, et al. Blood. 2011 May 12;117(19):5189-97. doi: 10.1182/blood-2010-09-299719. Epub 2011 Mar 17. Blood. 2011. PMID: 21415270 Free PMC article. - Alterations in platelet secretion differentially affect thrombosis and hemostasis.
Joshi S, Banerjee M, Zhang J, Kesaraju A, Pokrovskaya ID, Storrie B, Whiteheart SW. Joshi S, et al. Blood Adv. 2018 Sep 11;2(17):2187-2198. doi: 10.1182/bloodadvances.2018019166. Blood Adv. 2018. PMID: 30185436 Free PMC article. - The role of circulating platelet transcripts.
Clancy L, Freedman JE. Clancy L, et al. J Thromb Haemost. 2015 Jun;13 Suppl 1:S33-9. doi: 10.1111/jth.12922. J Thromb Haemost. 2015. PMID: 26149043 Review. - Transcript profiling of human platelets using microarray and serial analysis of gene expression (SAGE).
Gnatenko DV, Dunn JJ, Schwedes J, Bahou WF. Gnatenko DV, et al. Methods Mol Biol. 2009;496:245-72. doi: 10.1007/978-1-59745-553-4_16. Methods Mol Biol. 2009. PMID: 18839115 Review.
Cited by
- Expression of miRNA-26a in platelets is associated with clopidogrel resistance following coronary stenting.
Chen S, Qi X, Chen H, Li M, Gu J, Liu C, Xue H, Wang L, Geng Y, Qi P, Han Y. Chen S, et al. Exp Ther Med. 2016 Jul;12(1):518-524. doi: 10.3892/etm.2016.3278. Epub 2016 Apr 20. Exp Ther Med. 2016. PMID: 27347088 Free PMC article. - Modulation of Circulating MicroRNAs Levels during the Switch from Clopidogrel to Ticagrelor.
Carino A, De Rosa S, Sorrentino S, Polimeni A, Sabatino J, Caiazzo G, Torella D, Spaccarotella C, Mongiardo A, Strangio A, Filippis C, Indolfi C. Carino A, et al. Biomed Res Int. 2016;2016:3968206. doi: 10.1155/2016/3968206. Epub 2016 Jun 6. Biomed Res Int. 2016. PMID: 27366745 Free PMC article. Clinical Trial. - MicroRNAs in platelet function and cardiovascular disease.
McManus DD, Freedman JE. McManus DD, et al. Nat Rev Cardiol. 2015 Dec;12(12):711-7. doi: 10.1038/nrcardio.2015.101. Epub 2015 Jul 7. Nat Rev Cardiol. 2015. PMID: 26149483 Review. - MicroRNA-223 delivered by platelet-derived microvesicles promotes lung cancer cell invasion via targeting tumor suppressor EPB41L3.
Liang H, Yan X, Pan Y, Wang Y, Wang N, Li L, Liu Y, Chen X, Zhang CY, Gu H, Zen K. Liang H, et al. Mol Cancer. 2015 Mar 11;14:58. doi: 10.1186/s12943-015-0327-z. Mol Cancer. 2015. PMID: 25881295 Free PMC article. - Association of the Single Nucleotide Polymorphisms in microRNAs 130b, 200b, and 495 with Ischemic Stroke Susceptibility and Post-Stroke Mortality.
Kim J, Choi GH, Ko KH, Kim JO, Oh SH, Park YS, Kim OJ, Kim NK. Kim J, et al. PLoS One. 2016 Sep 7;11(9):e0162519. doi: 10.1371/journal.pone.0162519. eCollection 2016. PLoS One. 2016. PMID: 27603512 Free PMC article.
References
- Kottke-Marchant K. Importance of platelets and platelet response in acute coronary syndromes. Cleve Clin J Med. 2009;76 Suppl 1:S2–S7. - PubMed
- Hayward CP, Pai M, Liu Y, Moffat KA, Seecharan J, Webert KE, Cook RJ, Heddle NM. Diagnostic utility of light transmission platelet aggregometry: results from a prospective study of individuals referred for bleeding disorder assessments. J Thromb Haemost. 2009;7:676–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T15 HG000016-10S1/HG/NHGRI NIH HHS/United States
- R01 HL056652/HL/NHLBI NIH HHS/United States
- R01 HL102482-02/HL/NHLBI NIH HHS/United States
- R13 MH059864-01/MH/NIMH NIH HHS/United States
- T15 HG000016-11A1/HG/NHGRI NIH HHS/United States
- R01 HL088458/HL/NHLBI NIH HHS/United States
- T15 HG000016/HG/NHGRI NIH HHS/United States
- R01 HL088458-05/HL/NHLBI NIH HHS/United States
- R01 HL102482/HL/NHLBI NIH HHS/United States
- HL88458/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources