Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein - PubMed (original) (raw)
. 2009 Dec;10(6):499-506.
doi: 10.1016/j.cmet.2009.10.007.
Irena D Ignatova, Shin Yonemitsu, Yoshio Nagai, Paula Chatterjee, Dirk Weismann, Jennifer J Hsiao, Dongyan Zhang, Takanori Iwasaki, Romana Stark, Clare Flannery, Mario Kahn, Christopher M Carmean, Xing Xian Yu, Susan F Murray, Sanjay Bhanot, Brett P Monia, Gary W Cline, Varman T Samuel, Gerald I Shulman
Affiliations
- PMID: 19945407
- PMCID: PMC2799933
- DOI: 10.1016/j.cmet.2009.10.007
Prevention of hepatic steatosis and hepatic insulin resistance by knockdown of cAMP response element-binding protein
Derek M Erion et al. Cell Metab. 2009 Dec.
Abstract
In patients with poorly controlled type 2 diabetes mellitus (T2DM), hepatic insulin resistance and increased gluconeogenesis contribute to fasting and postprandial hyperglycemia. Since cAMP response element-binding protein (CREB) is a key regulator of gluconeogenic gene expression, we hypothesized that decreasing hepatic CREB expression would reduce fasting hyperglycemia in rodent models of T2DM. In order to test this hypothesis, we used a CREB-specific antisense oligonucleotide (ASO) to knock down CREB expression in liver. CREB ASO treatment dramatically reduced fasting plasma glucose concentrations in ZDF rats, ob/ob mice, and an STZ-treated, high-fat-fed rat model of T2DM. Surprisingly, CREB ASO treatment also decreased plasma cholesterol and triglyceride concentrations, as well as hepatic triglyceride content, due to decreases in hepatic lipogenesis. These results suggest that CREB is an attractive therapeutic target for correcting both hepatic insulin resistance and dyslipidemia associated with nonalcoholic fatty liver disease (NAFLD) and T2DM.
Conflict of interest statement
Conflict of interest: Xing Xian Yu, Susan F. Murray, Sanjay Bhanot, and Brett P. Monia own stock and/or hold stock options in Isis Pharmaceuticals.
Figures
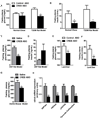
Figure 1. CREB ASO improves metabolic parameters in three other diabetic models
Fasting plasma glucose (A), and insulin (B) concentrations (n = 8–21) in the T2DM rat model. Fasting plasma glucose concentrations (C) were decreased with CREB ASO in the ZDF rat model (n = 4–5). Due to the pancreas saving effect of lower glucose values plasma insulin values tended to be increased (D) (n = 4–5). In the lard diet based model, CREB ASO reduced fasting plasma glucose (E), and insulin (F) concentrations (n = 7–9). Lastly, CREB ASO also reduced fasting plasma glucose in an ob/ob mouse model (G) due to reductions in gluconeogenic genes (H) (n = 4–5). * P < 0.05, ** P < 0.005.
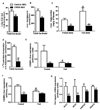
Figure 2. CREB ASO treatment reduces hepatic lipid content
Long chain acyl CoAs (A), and liver DAGs (B) were decreased with CREB ASO treatment (n = 8–10). Liver triglycerides were reduced in the fasted and fed states with CREB ASO (C). In-vivo lipogenesis was reduced with CREB ASO as assessed by the incorporation of [1-14C]-palmitate in to triglycerides (n = 4 per group) (D). C/EBPα (E), C/EBPβ (F), and key lipogenic genes (G) assessed by RT-PCR (n = 4–7). * P < 0.05, ** P < 0.005 for Control ASO vs. CREB ASO, # P < 0.05 for control ASO fasted vs. control ASO fed.
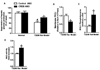
Figure 3. CREB ASO treatment improves hepatic insulin sensitivity in T2DM Rats
Suppression of hepatic glucose production (A) was assessed using the hyperinsulinemic-euglycemic clamp (n = 4–14 per treatment group). In the T2DM rat model CREB ASO reduced membrane PKCε (B) (n = 4 per treatment group), increased IRS-2 tyrosine phosphorylation (C) (n = 9 per treatment group), and improved Akt2 activity (D) (n = 5 per treatment group). * P < 0.05 for CREB ASO vs. control ASO, # P < 0.05 for T2DM control ASO vs. normal chow control ASO.
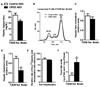
Figure 4. CREB ASO therapy decreases plasma triglycerides and cholesterol
Plasma triglycerides were decreased with CREB ASO (n = 10–12) (A). The lipoprotein profile showed significant reductions in VLDL, LDL, and HDL with CREB ASO (B). Free hepatic cholesterol was unchanged with CREB ASO (n = 4 per treatment group) (C) but CREB ASO decreased the concentration of hepatic cholesterol esters (D) as assessed by NMR. Synthesis of cholesterol in rat hepatocytes as assessed by relative [14C] acetic acid incorporation in to sterols (E) (n = 3–7 per treatment group). Amount of bile acids extracted from feces during a 12 hour fast (G) (n = 7 per treatment group). * P < 0.05 for control ASO vs. CREB ASO.
Similar articles
- Hepatic Sam68 Regulates Systemic Glucose Homeostasis and Insulin Sensitivity.
Qiao A, Ma W, Jiang Y, Han C, Yan B, Zhou J, Qin G. Qiao A, et al. Int J Mol Sci. 2022 Sep 29;23(19):11469. doi: 10.3390/ijms231911469. Int J Mol Sci. 2022. PMID: 36232770 Free PMC article. - Supplementation of persimmon leaf ameliorates hyperglycemia, dyslipidemia and hepatic fat accumulation in type 2 diabetic mice.
Jung UJ, Park YB, Kim SR, Choi MS. Jung UJ, et al. PLoS One. 2012;7(11):e49030. doi: 10.1371/journal.pone.0049030. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23145054 Free PMC article. - Endoplasmic reticulum-tethered transcription factor cAMP responsive element-binding protein, hepatocyte specific, regulates hepatic lipogenesis, fatty acid oxidation, and lipolysis upon metabolic stress in mice.
Zhang C, Wang G, Zheng Z, Maddipati KR, Zhang X, Dyson G, Williams P, Duncan SA, Kaufman RJ, Zhang K. Zhang C, et al. Hepatology. 2012 Apr;55(4):1070-82. doi: 10.1002/hep.24783. Epub 2012 Feb 9. Hepatology. 2012. PMID: 22095841 Free PMC article. - [Can the hyperactivity of lipogenesis cause hepatic steatosis? A role for ChREBP].
Robichon C, Girard J, Postic C. Robichon C, et al. Med Sci (Paris). 2008 Oct;24(10):841-6. doi: 10.1051/medsci/20082410841. Med Sci (Paris). 2008. PMID: 18950580 Review. French. - Regulation of hepatic glucose metabolism in health and disease.
Petersen MC, Vatner DF, Shulman GI. Petersen MC, et al. Nat Rev Endocrinol. 2017 Oct;13(10):572-587. doi: 10.1038/nrendo.2017.80. Epub 2017 Jul 21. Nat Rev Endocrinol. 2017. PMID: 28731034 Free PMC article. Review.
Cited by
- Transcriptional Regulation in Non-Alcoholic Fatty Liver Disease.
Steensels S, Qiao J, Ersoy BA. Steensels S, et al. Metabolites. 2020 Jul 9;10(7):283. doi: 10.3390/metabo10070283. Metabolites. 2020. PMID: 32660130 Free PMC article. Review. - Growth hormone abolishes beneficial effects of calorie restriction in long-lived Ames dwarf mice.
Gesing A, Al-Regaiey KA, Bartke A, Masternak MM. Gesing A, et al. Exp Gerontol. 2014 Oct;58:219-229. doi: 10.1016/j.exger.2014.08.010. Epub 2014 Aug 21. Exp Gerontol. 2014. PMID: 25152388 Free PMC article. - Reciprocal regulation of hepatic and adipose lipogenesis by liver X receptors in obesity and insulin resistance.
Beaven SW, Matveyenko A, Wroblewski K, Chao L, Wilpitz D, Hsu TW, Lentz J, Drew B, Hevener AL, Tontonoz P. Beaven SW, et al. Cell Metab. 2013 Jul 2;18(1):106-17. doi: 10.1016/j.cmet.2013.04.021. Cell Metab. 2013. PMID: 23823481 Free PMC article. - Prevention of diet-induced hepatic steatosis and hepatic insulin resistance by second generation antisense oligonucleotides targeted to the longevity gene mIndy (Slc13a5).
Pesta DH, Perry RJ, Guebre-Egziabher F, Zhang D, Jurczak M, Fischer-Rosinsky A, Daniels MA, Willmes DM, Bhanot S, Bornstein SR, Knauf F, Samuel VT, Shulman GI, Birkenfeld AL. Pesta DH, et al. Aging (Albany NY). 2015 Dec;7(12):1086-93. doi: 10.18632/aging.100854. Aging (Albany NY). 2015. PMID: 26647160 Free PMC article. - Effect of oxymatrine on liver gluconeogenesis is associated with the regulation of PEPCK and G6Pase expression and AKT phosphorylation.
Zhu YX, Hu HQ, Zuo ML, Mao L, Song GL, Li TM, Dong LC, Yang ZB, Ali Sheikh MS. Zhu YX, et al. Biomed Rep. 2021 Jul;15(1):56. doi: 10.3892/br.2021.1432. Epub 2021 Apr 27. Biomed Rep. 2021. PMID: 34007449 Free PMC article.
References
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
- Canettieri G, Koo SH, Berdeaux R, Heredia J, Hedrick S, Zhang X, Montminy M. Dual role of the coactivator TORC2 in modulating hepatic glucose output and insulin signaling. Cell Metab. 2005;2:331–338. - PubMed
- Cherrington AD, Stevenson RW, Steiner KE, Davis MA, Myers SR, Adkins BA, Abumrad NN, Williams PE. Insulin, glucagon, and glucose as regulators of hepatic glucose uptake and production in vivo. Diabetes Metab Rev. 1987;3:307–332. - PubMed
- Choi CS, Savage DB, Kulkarni A, Yu XX, Liu ZX, Morino K, Kim S, Distefano A, Samuel VT, Neschen S, et al. Suppression of diacylglycerol acyltransferase-2 (DGAT2), but not DGAT1, with antisense oligonucleotides reverses diet-induced hepatic steatosis and insulin resistance. J Biol Chem. 2007;282:22678–22688. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK049230/DK/NIDDK NIH HHS/United States
- L30 AG032893/AG/NIA NIH HHS/United States
- R01 DK-40936/DK/NIDDK NIH HHS/United States
- U24 DK059635/DK/NIDDK NIH HHS/United States
- P30 DK-45735/DK/NIDDK NIH HHS/United States
- R01 DK040936/DK/NIDDK NIH HHS/United States
- R24 DK085638/DK/NIDDK NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- P30 DK045735/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous