Experimental neonatal status epilepticus and the development of temporal lobe epilepsy with unilateral hippocampal sclerosis - PubMed (original) (raw)
Experimental neonatal status epilepticus and the development of temporal lobe epilepsy with unilateral hippocampal sclerosis
Mark Dunleavy et al. Am J Pathol. 2010 Jan.
Abstract
Hippocampal sclerosis is a common pathological finding in patients with temporal lobe epilepsy, including children, but a causal relationship to early-life seizures remains in question. Neonatal status epilepticus in animals can result in neuronal death within the hippocampus, although macroscopic features of hippocampal shrinkage are not evident at adulthood. Here, we examined electrophysiological and pathological consequences of focally evoked status epilepticus triggered by intra-amygdala microinjection of kainic acid in postnatal day 10 rat pups. Neonatal status epilepticus resulted in extensive neuronal death in the ipsilateral hippocampal CA1 and CA3 subfields and hilus, as assessed by DNA fragmentation and Fluoro-Jade B staining 72 hours later. The contralateral hippocampus was not significantly damaged. Histopathology at P55/P65 revealed unilateral hippocampal sclerosis (grade IV, modified Wyler/Watson scale) comprising >50% CA1 and CA3 neuron loss and astrogliosis. Additional features included hydrocephalus ex vacuo, modest dentate granule cell layer widening, and altered neuropeptide Y immunoreactivity indicative of synaptic rearrangement. Hippocampal atrophy was also evident on magnetic resonance imaging. Depth electrode recordings at adulthood detected spontaneous seizures that involved the ipsilateral hippocampus and amygdala. A significant positive correlation was found between hippocampal pathology grade and both frequency and duration of epileptic seizures at adulthood. The current study demonstrates that experimental neonatal status epilepticus can result in classical unilateral hippocampal sclerosis and temporal lobe epilepsy.
Figures
Figure 1
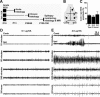
EEG and status epilepticus following intra-amygdala KA in P10 rats. A: Schematic of the experimental groups. Pups received intra-amygdala vehicle (veh) or KA at P10 and were analyzed at various times up to P150. B: Recording montage for acute bilateral EEG, depicting electrode placement over contralateral (contr. 1) and ipsilateral (ipsi. 2) cortex relative to the reference site (3). C: Graph showing lateralization of seizures in model as evidenced by lower EEG amplitude in contralateral recordings compared with ipsilateral signal strength for three sequential seizure events (averaged from n = 3 recordings). D and E: Representative EEG traces following intra-amygdala injection of 0.1 μg (D) and 2 μg (E) of KA, showing status epilepticus (continuous high-amplitude, high-frequency spiking) developing after injection of the high but not low dose of KA. Note seizure lateralization in E.
Figure 2
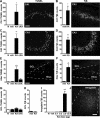
Permanent hippocampal damage 72 hours after neonatal status epilepticus in P10 rats. A–H: Quantification of ipsilateral TUNEL and FjB counts 72 hours after injection of vehicle (veh) or 2 μg of KA compared with age-matched naive (N) pups for CA1 (A and B), CA3 (C and D), hilus (E and F), and dentate granule cell layer (GCL) (G and H). Data from contralateral (c) fields are shown for 2-μg injected pups. Panels to the right of graphs are representative field views of TUNEL and FjB staining from 2-μg KA-injected pups at 72 hours. I: Quantification of FjB counts within the amygdala at the injection site for each dose tested. J: Representative photomicrograph of FjB staining within the ipsilateral amygdala at P13 in a 2-μg injected pup. Data are from n = 3–4 rats per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 compared with vehicle and naive pups. Scale bar in A–D, 100 μm; E and F, 50 μm; and J, 150 μm.
Figure 3
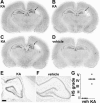
Unilateral hippocampal sclerosis at P55/P65 after neonatal status epilepticus in P10 pups. Representative nissl-stained sections from separate 2-μg KA-injected rats at P55 (A and B) and P65 (C), which underwent neonatal status epilepticus at P10. Note ipsilateral hippocampal sclerosis (arrows) and hydrocephalus ex vacuo of the lateral ventricle. D: Coronal section from a P55 rat that received intra-amygdala vehicle at P10. E and F: Representative photomicrographs (×2.5 lens) of ipsilateral hippocampus at P55 from rats that received intra-amygdala KA (2 μg) (E) and vehicle (F) at P10. Scale bar in E, 250 μm. G: Graph showing hippocampal sclerosis (HS) pathology scoring at P55 for rats given intra-amygdala vehicle (veh, n = 4) or KA (2 μg, n = 7) at P10.
Figure 4
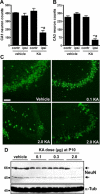
Hippocampal neuron loss at P55/65 following neonatal status epilepticus. A and B: Graphs depict NeuN-positive cell counts for contralateral (contr) and ipsilateral (ipsi) CA1 and CA3 subfields at P55 in rats given intra-amygdala vehicle or KA (2 μg) at P10 (n = 4–6 per group). Note significant loss of neurons in both subfields in rats subject to neonatal status epilepticus. ∗∗P < 0.01 versus ipsilateral vehicle control, #P < 0.05 versus contralateral KA. C: Fluorescence microscopy images (×20 lens) showing NeuN staining (green) of ipsilateral CA3 subfields of P55 rats that received intra-amygdala vehicle or KA (dose in micrograms) at P10. Note significant neuron loss in rats given 2 μg of KA. D: Western blot analysis at P65 of NeuN levels in hippocampal samples from vehicle- and KA-injected rats at P10 (n = 1 per lane). Note lower levels of NeuN protein in 2-μg KA-injected rats compared with other groups. α-Tubulin (α-Tub) is included as a protein loading control. Scale bar in C, 100 m.
Figure 5
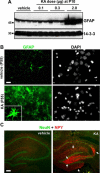
Hippocampal astrogliosis at P55/65 following neonatal status epilepticus. A: Western blot analysis at P65 of GFAP levels in hippocampal samples from rats given intra-amygdala vehicle or KA injection at P10 (n = 1 per lane). Note dramatically elevated GFAP in 2-μg KA-injected rats compared with other groups. 14-3-3 is included as a protein loading control. B: Fluorescence microscopy images (×40 lens) showing GFAP (green) staining of ipsilateral CA3 subfields of P55 rats that received either intra-amygdala vehicle or KA (2 μg) at P10. Note significant astrogliosis. Inset shows a higher power magnification of a single hypertrophic astrocyte. Nuclear morphology of cells is shown by 4′,6′-diamidino-2-phenylindole staining (DAPI) (gray). C: Representative photomicrographs showing hippocampus stained for NPY (red) and NeuN (green) at P55 in rats given either vehicle or KA (2 μg) at P10. NPY immunoreactivity demarcates hippocampal mossy fiber rearrangement (arrows). Scale bars in B, 12 μm; in C, 200 μm.
Figure 6
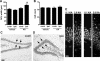
Granule cell layer widening at P55/65 following neonatal status epilepticus. A: Graph showing granule cell layer widths. Significant widening was detected in ipsilateral (ipsi) hippocampus of 2-μg KA-injected pups by adulthood (n = 4–6 per group). ∗P < 0.05 versus ipsilateral side of vehicle control; #P < 0.05 versus contralateral (contr) side of KA injected. B: Graph of granule cell layer counts from the same area show no differences between groups. C and D: Representative photomicrographs of the contralateral and ipsilateral dentate gyrus of a status epilepticus rat at P55 with widening of layer on ipsilateral side. Arrows highlight area of comparison. E: Representative photomicrographs of the upper blade of the ipsilateral dentate granule cell layer from 0.1- and 2-μg KA-injected rats at P55 at two different positions. Note difference in layer width despite numbers of cells being comparable. Scale bars in C, 100 μm; in E, 15 μm.
Figure 7
Amygdala injection site at P65. A: Graph showing NeuN counts within the ipsilateral amygdala of vehicle (veh)- and 2-μg KA-injected rats at P65 (n = 3–4 per group). ∗P < 0.05 compared with vehicle group. B: Representative double-label fluorescence immunostaining (×40 lens) of NeuN and GFAP in sections from P65 rats at the site corresponding to the intraamygala injection point at P10. Scale bar, 12 μm.
Figure 8
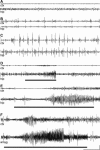
Spontaneous temporal lobe seizures at adulthood in rats subject to neonatal status epilepticus. A–E: Representative EEG traces from cortex (ctx) and hippocampal (hip) electrodes in adult rats following intra-amygdala injection at P10 of vehicle (A) or 2 μg of KA (B–E). A: Trace from a rat (P133) that received vehicle at P10. B: Interictal events associated with no alteration in behavior. C: High-amplitude, low-frequency electrographic events in hippocampus and cortex associated with periods of immobility and intermittent myoclonus. Brief (D) and prolonged (E) spontaneous electrographic seizures (high-amplitude, high-frequency spiking) recorded in the hippocampus. F: Representative spontaneous seizure captured in a P120 rat that received 2 μg of KA at P10 during combined intra-amygdala (amyg) and cortical EEG recordings. The bold lines represent the period of behavioral seizure (greater than Racine scale 3). Scale bar: voltage, 200 μV (A–F); time, 20 seconds (A–C) and 10 seconds (D–F).
Figure 9
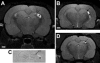
MRI confirmation of hippocampal sclerosis in epileptic rats at adulthood. A: MRI scan (7T) at the level of dorsal hippocampus of an epileptic rat at P150 that underwent neonatal status epilepticus, in which unilateral hippocampal sclerosis is evident (arrow). Asterisk denotes signal from hypertrophied ventrical. B: MRI from same epileptic rat in A at a more ventral level. C: Corresponding histology from the imaged animal with same features denoted. D: Imaged separate epileptic rat confirming interanimal similarity in sclerosis as resolved by MRI. Bar in A and B, 1 mm; in C and D, 0.75 mm.
Similar articles
- Progression of neuronal damage after status epilepticus and during spontaneous seizures in a rat model of temporal lobe epilepsy.
Pitkänen A, Nissinen J, Nairismägi J, Lukasiuk K, Gröhn OH, Miettinen R, Kauppinen R. Pitkänen A, et al. Prog Brain Res. 2002;135:67-83. doi: 10.1016/S0079-6123(02)35008-8. Prog Brain Res. 2002. PMID: 12143371 - The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy.
Sloviter RS. Sloviter RS. Ann Neurol. 1994 Jun;35(6):640-54. doi: 10.1002/ana.410350604. Ann Neurol. 1994. PMID: 8210220 Review. - Status epilepticus in epileptogenesis.
Kapur J. Kapur J. Curr Opin Neurol. 1999 Apr;12(2):191-5. doi: 10.1097/00019052-199904000-00010. Curr Opin Neurol. 1999. PMID: 10226752 Review.
Cited by
- Hippocampal body changes in pure partial onset sleep and pure partial onset waking epileptic patients.
Motamedi M, Zandieh A, Hajimirzabeigi A, Tahsini M, Vakhshiteh F, Rahimian E. Motamedi M, et al. Neurol Sci. 2013 Sep;34(9):1529-35. doi: 10.1007/s10072-012-1275-7. Epub 2013 Jan 3. Neurol Sci. 2013. PMID: 23283529 - The kainic acid model of temporal lobe epilepsy.
Lévesque M, Avoli M. Lévesque M, et al. Neurosci Biobehav Rev. 2013 Dec;37(10 Pt 2):2887-99. doi: 10.1016/j.neubiorev.2013.10.011. Epub 2013 Oct 30. Neurosci Biobehav Rev. 2013. PMID: 24184743 Free PMC article. Review. - Local disruption of glial adenosine homeostasis in mice associates with focal electrographic seizures: a first step in epileptogenesis?
Li T, Lytle N, Lan JQ, Sandau US, Boison D. Li T, et al. Glia. 2012 Jan;60(1):83-95. doi: 10.1002/glia.21250. Epub 2011 Sep 30. Glia. 2012. PMID: 21964979 Free PMC article. - A novel, noninvasive, predictive epilepsy biomarker with clinical potential.
Choy M, Dubé CM, Patterson K, Barnes SR, Maras P, Blood AB, Hasso AN, Obenaus A, Baram TZ. Choy M, et al. J Neurosci. 2014 Jun 25;34(26):8672-84. doi: 10.1523/JNEUROSCI.4806-13.2014. J Neurosci. 2014. PMID: 24966369 Free PMC article. - Ghrelin improves pilocarpine‑induced cerebral cortex inflammation in epileptic rats by inhibiting NF‑κB and TNF‑α.
Han K, Wang QY, Wang CX, Luan SY, Tian WP, Wang Y, Zhang RY. Han K, et al. Mol Med Rep. 2018 Oct;18(4):3563-3568. doi: 10.3892/mmr.2018.9381. Epub 2018 Aug 10. Mol Med Rep. 2018. PMID: 30106107 Free PMC article.
References
- Mathern GW, Babb TL, Armstrong DL. Hippocampal Sclerosis In Epilepsy: A Comprehensive Textbook. In: Engel JJ, Pedley TA, editors. Lippincott-Raven Publishers; Philadelphia: 1997. p. 133.
- Meldrum BS, Bruton CJ. Epilepsy. In: Adams JH, Duchen LW, editors. Oxford University Press; New York: 1992. p. 1246.
- Guerrini R. Epilepsy in children. Lancet. 2006;367:499–524. - PubMed
- Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990;535:195–204. - PubMed
- Davies KG, Hermann BP, Dohan FC, Jr, Foley KT, Bush AJ, Wyler AR. Relationship of hippocampal sclerosis to duration and age of onset of epilepsy, and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res. 1996;24:119–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous