Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors - PubMed (original) (raw)
Clinical Trial
. 2010 Jan 10;28(2):207-14.
doi: 10.1200/JCO.2009.22.9237. Epub 2009 Nov 30.
Mace L Rothenberg, Jakob Dupont, Wendy Cooper, Paul Chevalier, Lars Sternas, Giliane Buzenet, Elizabeth Koehler, Jeffrey A Sosman, Lawrence H Schwartz, David H Gultekin, Jason A Koutcher, Edwin F Donnelly, Ric Andal, Isabelle Dancy, David R Spriggs, William P Tew
Affiliations
- PMID: 19949018
- PMCID: PMC2815710
- DOI: 10.1200/JCO.2009.22.9237
Clinical Trial
Phase I study of intravenous vascular endothelial growth factor trap, aflibercept, in patients with advanced solid tumors
A Craig Lockhart et al. J Clin Oncol. 2010.
Abstract
Purpose: Vascular endothelial growth factor (VEGF) Trap (aflibercept) is an angiogenesis inhibitor comprising portions of the extracellular domains of human VEGF receptors 1 and 2 fused to the Fc portion of human immunoglobulin G. This phase I study was designed to evaluate the safety, pharmacokinetics, and pharmacodynamics of VEGF Trap administered intravenously (IV) every 2 weeks.
Patients and methods: Patients with refractory solid tumors or non-Hodgkin's lymphoma with adequate organ function were eligible. Pharmacokinetic/pharmacodynamic markers included measurement of plasma VEGF bound to VEGF Trap and free VEGF Trap. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was incorporated to measure the biologic effects of the drug on tumor vascularity and permeability.
Results: The study enrolled 47 patients at doses ranging from 0.3 to 7.0 mg/kg IV every 2 weeks. Dose-limiting toxicities were rectal ulceration and proteinuria at the 7.0 mg/kg dose. Other mechanism-specific toxicities included hypertension. On the basis of these observations and on pharmacokinetics, the recommended phase II dose of VEGF Trap as a single agent is 4 mg/kg every 2 weeks. Three RECIST (Response Evaluation Criteria in Solid Tumors) -defined partial responses were observed, one at the 3.0 mg/kg and two at the 7.0 mg/kg dose level. Maximum plasma concentration of free VEGF Trap increased proportionally with dose. Maximal VEGF-bound VEGF Trap complex levels were reached at doses > or = 2.0 mg/kg. Changes in volume transfer constant measured by DCE-MRI at baseline and at 24 hours after administration indicate a possible dose-related change in this pharmacodynamic marker.
Conclusion: IV VEGF Trap was well tolerated at the dose levels tested. Pharmacodynamic and pharmacokinetic markers were indicative of VEGF blockade.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
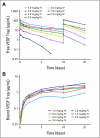
Fig 1.
Mean plasma concentration versus time profiles for (A) free and (B) bound vascular endothelial growth factor (VEGF) Trap (log scale). (A) After the first two doses (on days 0 and 14) of intravenous (IV) VEGF Trap, there is a dose-dependent increase in free VEGF Trap maximum plasma concentration, and detectable levels are present at doses of 2.0 mg/kg and greater. (B) At dose levels of 2.0 mg/kg and greater, bound VEGF Trap levels appear to saturate and have no appreciable increase with dose escalation.
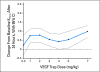
Fig 2.
Mean change in volume transfer constant (Ktrans) between baseline and 24 hours for each vascular endothelial growth factor (VEGF) Trap dose estimated from a linear analysis of covariance model. The average dose effects (points) and 95% CI (dotted lines) are shown. The decrease in Ktrans after 24 hours was significant at all doses except 0.3 and 4.0 mg/kg. Baseline and 24-hour Ktrans measurements were significantly different between the 0.3 and 7.0 mg/kg doses (0.98 v 0.22; P = .0276).
Comment in
- Hunting and trapping the vascular endothelial growth factor.
Dowlati A. Dowlati A. J Clin Oncol. 2010 Jan 10;28(2):185-7. doi: 10.1200/JCO.2009.25.4359. Epub 2009 Nov 30. J Clin Oncol. 2010. PMID: 19949005 No abstract available. - Biomarkers of antiangiogenic therapy: how do we move from candidate biomarkers to valid biomarkers?
Duda DG, Ancukiewicz M, Jain RK. Duda DG, et al. J Clin Oncol. 2010 Jan 10;28(2):183-5. doi: 10.1200/JCO.2009.24.8021. Epub 2009 Nov 30. J Clin Oncol. 2010. PMID: 19949009 No abstract available.
Similar articles
- Phase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumors.
Tew WP, Gordon M, Murren J, Dupont J, Pezzulli S, Aghajanian C, Sabbatini P, Mendelson D, Schwartz L, Gettinger S, Psyrri A, Cedarbaum JM, Spriggs DR. Tew WP, et al. Clin Cancer Res. 2010 Jan 1;16(1):358-66. doi: 10.1158/1078-0432.CCR-09-2103. Epub 2009 Dec 22. Clin Cancer Res. 2010. PMID: 20028764 Free PMC article. Clinical Trial. - Phase I dose-escalation study of the safety, tolerability, and pharmacokinetics of aflibercept in combination with S-1 in Japanese patients with advanced solid malignancies.
Doi T, Boku N, Onozawa Y, Takahashi K, Kawaguchi O, Ohtsu A. Doi T, et al. Invest New Drugs. 2020 Oct;38(5):1390-1399. doi: 10.1007/s10637-019-00888-z. Epub 2020 Jan 6. Invest New Drugs. 2020. PMID: 31907738 Free PMC article. Clinical Trial. - Phase I Dose-Escalation and Pharmacokinetic Study of Intravenous Aflibercept in Combination with Docetaxel, Cisplatin, and 5-Fluorouracil in Patients with Advanced Solid Malignancies.
Bahleda R, Baker J, Massard C, Gadgeel SM, Rogers JE, Izzedine H, Deutsch E, Garris JL, Khan A, Boelle E, Assadourian S, Soria JC, Ajani JA. Bahleda R, et al. Oncology. 2016;90(1):10-20. doi: 10.1159/000440958. Epub 2015 Oct 23. Oncology. 2016. PMID: 26492090 Clinical Trial. - Aflibercept (VEGF Trap): one more double-edged sword of anti-VEGF therapy for cancer?
Jin K, Shen Y, He K, Xu Z, Li G, Teng L. Jin K, et al. Clin Transl Oncol. 2010 Aug;12(8):526-32. doi: 10.1007/s12094-010-0550-4. Clin Transl Oncol. 2010. PMID: 20709650 Review. - Clinical applications of VEGF-trap (aflibercept) in cancer treatment.
Teng LS, Jin KT, He KF, Zhang J, Wang HH, Cao J. Teng LS, et al. J Chin Med Assoc. 2010 Sep;73(9):449-56. doi: 10.1016/S1726-4901(10)70097-6. J Chin Med Assoc. 2010. PMID: 20875616 Review.
Cited by
- Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements.
Akter S, Rahman MA, Hasan MN, Akhter H, Noor P, Islam R, Shin Y, Rahman MDH, Gazi MS, Huda MN, Nam NM, Chung J, Han S, Kim B, Kang I, Ha J, Choe W, Choi TG, Kim SS. Akter S, et al. Cells. 2022 Feb 13;11(4):650. doi: 10.3390/cells11040650. Cells. 2022. PMID: 35203301 Free PMC article. Review. - Monoclonal antibodies and chimeric antigen receptor (CAR) T cells in the treatment of colorectal cancer.
Jin KT, Chen B, Liu YY, Lan HU, Yan JP. Jin KT, et al. Cancer Cell Int. 2021 Feb 1;21(1):83. doi: 10.1186/s12935-021-01763-9. Cancer Cell Int. 2021. PMID: 33522929 Free PMC article. Review. - Advances in radiotherapy and comprehensive treatment of high-grade glioma: immunotherapy and tumor-treating fields.
Liu S, Zhao Q, Shi W, Zheng Z, Liu Z, Meng L, Dong L, Jiang X. Liu S, et al. J Cancer. 2021 Jan 1;12(4):1094-1104. doi: 10.7150/jca.51107. eCollection 2021. J Cancer. 2021. PMID: 33442407 Free PMC article. Review. - Nucleic Acid-Based Approaches for Tumor Therapy.
Hager S, Fittler FJ, Wagner E, Bros M. Hager S, et al. Cells. 2020 Sep 9;9(9):2061. doi: 10.3390/cells9092061. Cells. 2020. PMID: 32917034 Free PMC article. Review. - Inflammation- and angiogenesis-related biomarkers are correlated with cancer-related fatigue in colorectal cancer patients: Results from the ColoCare Study.
Himbert C, Ose J, Lin T, Warby CA, Gigic B, Steindorf K, Schrotz-King P, Abbenhardt-Martin C, Zielske L, Boehm J, Ulrich CM. Himbert C, et al. Eur J Cancer Care (Engl). 2019 Jul;28(4):e13055. doi: 10.1111/ecc.13055. Epub 2019 Apr 24. Eur J Cancer Care (Engl). 2019. PMID: 31016796 Free PMC article. Clinical Trial.
References
- Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. - PubMed
- Senger DR, Perruzzi CA, Feder J, et al. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. - PubMed
- Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources