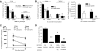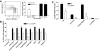CD70 expression by dendritic cells plays a critical role in the immunogenicity of CD40-independent, CD4+ T cell-dependent, licensed CD8+ T cell responses - PubMed (original) (raw)
CD70 expression by dendritic cells plays a critical role in the immunogenicity of CD40-independent, CD4+ T cell-dependent, licensed CD8+ T cell responses
Katherine E Van Deusen et al. J Leukoc Biol. 2010 Mar.
Abstract
The stimulation of DC by CD4(+) T cells is known to condition DC to activate naïve CD8(+) T cells, predominantly via CD40-CD40L interactions. It has been proposed that a critical consequence of DC conditioning is the induction of CD70 expression. Whether and how CD70 induction contributes to CD8(+) T cell responses in the absence of CD40-CD40L interactions are unknown. CD8(+) T cell responses to adenoviral- or DC-based immunization of CD40-deficient mice revealed a CD40-independent, CD4(+) T cell-dependent pathway for CD70 induction on conventional DC. This pathway and subsequent CD8(+) T cell responses were enhanced by, but not dependent on, concomitant activation of TLR and in part, used TRANCE and LIGHT/LTalphabeta stimulation. Blocking TRANCE and LIGHT/LTalphabeta during stimulation reduced the immunogenicity of CD40-deficient DC. These data support the hypothesis that induction of CD70 expression on DC after an encounter with activated CD4(+) T cells is a major component of CD4(+) T cell-mediated licensing of DC. Further, multiple pathways exist for CD4(+) T cells to elicit CD70 expression on DC. These data in part explain the capacity of CD40-deficient mice to mount CD8(+) T cell responses and may provide additional targets for immunotherapy in situations when CD40-mediated licensing is compromised.
Figures
Figure 1.
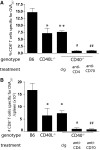
CD40-independent CD8+ T cell responses are dependent on CD70 and CD4+ T cells. (A) Wild-type B6, CD40−/−, and CD40L−/− mice were injected i.v. with 2 × 108 PFU of OVA-adeno. CD40−/− mice were treated with cIg, anti-CD4, or anti-CD70 as described in Materials and Methods. Seven days later, the magnitude of the primary CD8+ T cell response was assessed, staining splenocytes from infected mice with anti-CD8, anti-CD44, and OVA257-tetramer. (B) Number of OVA257-specific CD8+ T cells in the spleens of the indicated mice immunized and treated as described in A. *, P < 0.05; **, P < 0.01, compared with B6; #, P < 0.05; ##, P < 0.01, compared with cIg-treated CD40−/−. The experiments were performed twice with similar results.
Figure 2.
DC activated independently of CD40-CD40L are dependent on CD70 for immunogenicity. BMDC generated from wild-type or CD40−/− mice were cococultured with OT-II CD4+ T cells for 48 h in the presence or absence of 10 μg/ml OVA323. (A) Immunogenicity of wild-type and CD40−/− BMDC. Enriched BMDC from OT-II cocultures containing OVA323 (solid bars) or no peptide (open bars) were pulsed with 10 μg/ml OVA257 peptide and 1 × 105 used to immunize B6 mice. Seven days after immunization, lungs or spleen from mice were harvested and stained for OVA257-specific CD8+ T cells. *, P < 0.05; **, P < 0.01, compared with B6 cultures containing OVA323; #, P < 0.05, compared with CD40−/− DC cocultured with OVA323. (B) Blockade of CD70 on CD40-independently activated BMDC minimizes immunogenicity. BMDC, derived as above, were pulsed with OVA257 and 100 μg/ml cIg (solid bars) or anti-CD70 (open bars) for 2 h, and 1 × 105 were injected i.v. into B6 mice. The frequency (upper panel) and number (lower panel) of OVA257-specific CD8+ T cells in the lungs (upper panel) and spleen (upper and lower panels) 7 days after immunization were determined by CD8 and MHC-tetramer staining. *, P < 0.05; **, P < 0.01, compared with B6 BMDC pulsed with cIg; #, P < 0.05; ##, P < 0.01, compared with CD40−/− BMDC pulsed with cIg. (C) Anti-CD70 treatment does not enhance clearance of BMDC. CFSE-labeled BMDC (2×105) were treated with cIg or anti-CD70 and transferred into B6 recipients. The number of CFSE+CD11c+ DC was counted at 24 h and 48 h. Experiments were repeated once with equivalent results. PTLN, Paratracheal lymph node. (D) Frequency of OVA257-specific CD8+ T cell response in lungs of B6 or CD40−/− mice that received OT-II cells and then immunized with OVA323 and OVA257. CD40-deficient mice received cIg or anti-CD70 (500 μg i.p.) as indicated. *, P < 0.05; **, P < 0.01, compared with B6 mice receiving OVA323; ##, P < 0.01, compared with CD40−/− mice receiving OVA323 and cIg.
Figure 3.
CD40L-independent licensing of CD8+ T cell responses to peptide does not require MyD88-mediated stimulation. B6 or MyD88-deficient mice received CD40L-deficient OT-II cells and were challenged with OVA257 and HBC128 or OVA323 and cIg or anti-CD70. The magnitude of CD8+ T cell response to OVA257 was determined by MHC-tetramer and CD8 staining of splenocytes 7 days later. The experiment was repeated twice.
Figure 4.
CD4+ T cells can induce CD70 expression on conventional DC independently from CD40-CD40L stimulation. (A) Flow cytometry gating strategy for DC; live lymphocytes were further negatively gated for CD19 and CD3 and positively gated for CD11c expression. SSC, Side-scatter; FSC, forward-scatter. (B) Specific induction of CD70 on DC. In vitro cultures were established with enriched OT-II CD4+ T cells and splenocytes/lymph node cells with OVA323 peptide or HBC128. Forty-eight hours later, cultures were analyzed by flow cytometry for the up-regulation of CD86 and CD70 on CD11c+ cells. (C) Direct induction of CD70 expression on DC by CD4+ T cells. Enriched OT-II CD4+ T cells were cultured with purified DC and OVA323 peptide. (Left panel) Identification of conventional DC subsets by expression of CD8α or CD11b. (Middle and right panels) Expression of CD86 and CD70 on conventional DC subsets. (D) CD70 induction without CD40-CD40L stimulation. Cultures of OT-II cells and B6 or CD40-deficient splenocytes were established with OVA323 or HBC128. Cultures with B6 splenocytes included cIg or anti-CD40L (100 μg/ml). *, P < 0.05, comparing OVA323-induced responses and HBC128-induced responses. (E) B6 or CD40-deficient mice received OT-II or CD40L-deficient OT-II CD4+ T cells and were challenged with PBS, HBC128, or OVA323 peptide (500 μg). Expression of CD70 on CD3/CD19−CD11c+ DC was determined 48 h later. Data in quadrants indicate percentage of CD3/CD19−CD11c+ cells staining with CD70. *, P < 0.05, comparing OVA323-induced responses and HBC128-induced responses. Data are representative of three similar experiments.
Figure 5.
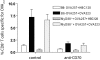
TLR signals enhance CD70 induction on DC by CD4+ T cells but are not required. (A) Induction of CD70 in vivo does not require MyD88. OT-II were transferred into B6 or MyD88-deficient mice, challenged with HBC128 or OVA23 peptide, and analyzed for CD86 and CD70 expression on CD11c+ cells. Plots are derived from single mice. (B) Enhanced CD70 expression after TLR signaling. OT-II cells were transferred into recipient B6 mice and then challenged with HBC128, PAM3CSK4, OVA323, or OVA323 + PAM3CSK4. Splenocyte populations were stained for CD70 and CD86 expression on CD3/CD19−CD11c+ DC. (C) Concomitant TLR stimulation enhances sensitivity of CD70-dependent CD8+ T cell responses to OVA257. OT-II were transferred into recipient mice and challenged with the indicated amounts of OVA257 and PAM3CSK4 or PBS. The magnitude of the OVA257-specific CD8+ T cell responses was determined by CD8 and MHC-tetramer staining of spleens 7 days later. Anti-CD70-treated mice served as controls (striped and checkered bars, respectively). *, P < 0.05; **, P < 0.01, when compared with OVA323 + PAM3CSK4-induced responses. (D) Concomitant TLR stimulation reduces the frequency of CD4+ T cells necessary to support CD8+ T cell response to OVA257. Staggered amounts of OT-II cells were transferred into recipient B6 mice and then challenged with OVA257 and PAM3CSK4 or PBS. Seven days later, OVA257-specific CD8+ T cells were detected in the spleens as described above. Error bars indicate
sem
of three mice/group. Anti-CD70-treated mice served as controls (striped and checkered bars, respectively). *, P < 0.05; **, P < 0.01, when compared with OVA323 + PAM3CSK4-induced responses. Experiments in A and B were repeated three times and C and D twice, with similar results. Dashed line indicates limited detection of significant responses using MHC tetramer (∼0.5%). Arrow indicates data points that are the first at which a significant positive response is detected.
Figure 6.
Contribution of LTβR and TRANCE to the expression of CD70 on DC in the absence of CD40. MHC class II+ (CD45.1+) or MHC class II− (CD45.2+) splenocytes were mixed 1:1 in coculture with OT-II T cells and OVA323 peptide or with CD40L-expressing 3T3 cells. Forty-eight hours after culture initiation, splenoctyes were stained for CD70 expression on CD11c+ DC. (A) Dot plots (gated on CD11c+ cells) show MHC class II+ and class II− DC. Bar chart indicates the proportion of MHC class II+ or class II− DC induced to express CD70 by activated CD4+ T cells or CD40L-expressing fibroblasts. (B) CD86 and CD70 expression on B6 or CD40–CD11c+ DC after 48 h coculture with OT-II cells and OVA323 in the presence or absence of blocking antibodies against TRANCE and LAG-3 and the chimeric protein LTβR-Ig. (A) Representative of one of two experiments; (B) representative of three similar experiments. *, P < 0.05, when compared with unblocked, CD40-deficient DC. (C) Immunogenicity of CD40− DC after blockade of LTβR and TRANCE. MHC-tetramer staining of primary CD8+ T cell responses from mice immunized with OVA257-pulsed DC isolated from cultures of B6 or CD40-deficient splenocytes with OT-II, as described in Materials and Methods, and treated with cIg or anti-CD70 prior to immunization. *, P < 0.05, compared with B6 DC; **, P < 0.01, compared with cIg-treated, CD40-deficient DC; #, P < 0.05, compared with cIg-treated, CD40-deficient DC. Experiment was repeated twice.
Similar articles
- Comparison of OX40 ligand and CD70 in the promotion of CD4+ T cell responses.
Kurche JS, Burchill MA, Sanchez PJ, Haluszczak C, Kedl RM. Kurche JS, et al. J Immunol. 2010 Aug 15;185(4):2106-15. doi: 10.4049/jimmunol.1000172. Epub 2010 Jul 16. J Immunol. 2010. PMID: 20639485 Free PMC article. - CD8+ dendritic cell-mediated tolerance of autoreactive CD4+ T cells is deficient in NOD mice and can be corrected by blocking CD40L.
Price JD, Beauchamp NM, Rahir G, Zhao Y, Rieger CC, Lau-Kilby AW, Tarbell KV. Price JD, et al. J Leukoc Biol. 2014 Feb;95(2):325-36. doi: 10.1189/jlb.0113013. Epub 2013 Sep 30. J Leukoc Biol. 2014. PMID: 24082013 Free PMC article. - CD40 and dendritic cell function.
O'Sullivan B, Thomas R. O'Sullivan B, et al. Crit Rev Immunol. 2003;23(1-2):83-107. doi: 10.1615/critrevimmunol.v23.i12.50. Crit Rev Immunol. 2003. PMID: 12906261 Review. - The critical role of CD40/CD40L in the CD4-dependent generation of CD8+ T cell immunity.
Clarke SR. Clarke SR. J Leukoc Biol. 2000 May;67(5):607-14. doi: 10.1002/jlb.67.5.607. J Leukoc Biol. 2000. PMID: 10810999 Review.
Cited by
- Requirement of CD4 help for induction of CD8 T cell response specific for virally derived h60.
Ryu SJ, Kang B, Kim SH, Kim TW, Chang J, Choi EY. Ryu SJ, et al. Immune Netw. 2012 Jun;12(3):118-25. doi: 10.4110/in.2012.12.3.118. Epub 2012 Jun 30. Immune Netw. 2012. PMID: 22916048 Free PMC article. - Diacylglycerol Lipase-β Is Required for TNF-α Response but Not CD8+ T Cell Priming Capacity of Dendritic Cells.
Shin M, Buckner A, Prince J, Bullock TNJ, Hsu KL. Shin M, et al. Cell Chem Biol. 2019 Jul 18;26(7):1036-1041.e3. doi: 10.1016/j.chembiol.2019.04.002. Epub 2019 May 16. Cell Chem Biol. 2019. PMID: 31105063 Free PMC article. - CD70 and IFN-1 selectively induce eomesodermin or T-bet and synergize to promote CD8+ T-cell responses.
Dong H, Franklin NA, Ritchea SB, Yagita H, Glennie MJ, Bullock TN. Dong H, et al. Eur J Immunol. 2015 Dec;45(12):3289-301. doi: 10.1002/eji.201445291. Epub 2015 Nov 6. Eur J Immunol. 2015. PMID: 26461455 Free PMC article. - Quality controls in cellular immunotherapies: rapid assessment of clinical grade dendritic cells by gene expression profiling.
Castiello L, Sabatino M, Zhao Y, Tumaini B, Ren J, Ping J, Wang E, Wood LV, Marincola FM, Puri RK, Stroncek DF. Castiello L, et al. Mol Ther. 2013 Feb;21(2):476-84. doi: 10.1038/mt.2012.89. Epub 2012 Nov 13. Mol Ther. 2013. PMID: 23147403 Free PMC article. - Stimulating CD27 to quantitatively and qualitatively shape adaptive immunity to cancer.
Bullock TN. Bullock TN. Curr Opin Immunol. 2017 Apr;45:82-88. doi: 10.1016/j.coi.2017.02.001. Epub 2017 Mar 17. Curr Opin Immunol. 2017. PMID: 28319731 Free PMC article. Review.
References
- Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. - PubMed
- Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. - PubMed
- Mescher M F, Curtsinger J M, Agarwal P, Casey K A, Gerner M, Hammerbeck C D, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials