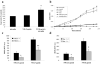Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson's disease - PubMed (original) (raw)
Comparative Study
doi: 10.1007/s12640-009-9140-z. Epub 2009 Dec 3.
Kester Phillips, Albert R Wielgus, Jie Liu, Alberto Albertini, Fabio A Zucca, Rudolph Faust, Steven Y Qian, David S Miller, Colin F Chignell, Belinda Wilson, Vernice Jackson-Lewis, Serge Przedborski, Danielle Joset, John Loike, Jau-Shyong Hong, David Sulzer, Luigi Zecca
Affiliations
- PMID: 19957214
- PMCID: PMC3603276
- DOI: 10.1007/s12640-009-9140-z
Comparative Study
Neuromelanin activates microglia and induces degeneration of dopaminergic neurons: implications for progression of Parkinson's disease
Wei Zhang et al. Neurotox Res. 2011 Jan.
Abstract
In Parkinson's disease (PD), there is a progressive loss of neuromelanin (NM)-containing dopamine neurons in substantia nigra (SN) which is associated with microgliosis and presence of extracellular NM. Herein, we have investigated the interplay between microglia and human NM on the degeneration of SN dopaminergic neurons. Although NM particles are phagocytized and degraded by microglia within minutes in vitro, extracellular NM particles induce microglial activation and ensuing production of superoxide, nitric oxide, hydrogen peroxide (H₂O₂), and pro-inflammatory factors. Furthermore, NM produces, in a microglia-depended manner, neurodegeneration in primary ventral midbrain cultures. Neurodegeneration was effectively attenuated with microglia derived from mice deficient in macrophage antigen complex-1, a microglial integrin receptor involved in the initiation of phagocytosis. Neuronal loss was also attenuated with microglia derived from mice deficient in phagocytic oxidase, a subunit of NADPH oxidase, that is responsible for superoxide and H₂O₂ production, or apocynin, an NADPH oxidase inhibitor. In vivo, NM injected into rat SN produces microgliosis and a loss of tyrosine hydroxylase neurons. Thus, these results show that extracellular NM can activate microglia, which in turn may induce dopaminergic neurodegeneration in PD. Our study may have far-reaching implications, both pathogenic and therapeutic.
Figures
Fig. 1. Microglia activated by NM produce iROS
(a) NM enhances microglial superoxide production, as measured by electron spin resonance (mean ± s.e.m.; n = 3; * p < 0.05). (b) Measurement of H2O2 production from microglial cultures over time indicate that NM (5 μg/ml) elicits H2O2 production. This is effectively blocked by the PHOX inhibitor, apocyanin (100 μM). H2O2 synthesis is triggered by PMA (100 μM), a known PHOX activator (mean ± s.e.m.; n = 3). (c, d) DCF fluorescence in response to NM, as an indicator of iROS production. (c) PHOX−/− mice produce less iROS in response to NM (mean ± s.e.m.; n = 8; * p < 0.05; ** p < 0.01). (d) Mac-1−/− microglia produce less iROS in response to NM (mean ± s.e.m.; n = 6; * p < 0.05).
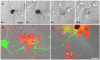
Fig. 2. Microglia phagocytose and degrade NM
(a) Series of differential contrast images of microglial phagocytosis and degradation of a large particle (~ 14 nm diameter) of NM in ventral midbrain / astrocyte / microglial co-culture at 2 h intervals excerpted from a video
http://www.sulzerlab.org/videos/NMmicrogliaDIC.mov
. Image acquisition commenced 15 min after addition of NM for 10 h. A microglial cell to the right of the particle at 0 h is in contact with the particle at 2 h. At 4 h, the pigment appears to be absent or nearly so and additional microglia have arrived at the scene. Scale bar = 20 μm. For an example of uptake of NM by microglial filipodia and subsequent NM degradation, see
http://www.sulzerlab.org/videos/NMfilopodia.mov
. (b, c) The co-cultures prepared as above were fixed 72 h after addition of NM and immunolabeled for dopaminergic neurons by TH (green) and activated microglia by OX-42 (red). Examples of NM particles that have been phagocytosed are indicated by the single-headed arrows. The double-headed arrow indicates an example of a swollen neurite varicosity, which is several-fold larger than typical varicosities in these neurons (average ~1.2 μm, Pothos et al. 1998) which can also be observed. The swollen varicosities are an indicator of toxicity (Cubells et al. 1994). Scale bar = 20 μm.
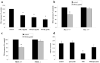
Fig. 3. Neuronal death induced by NM-activated microglia
(a) NM is toxic to dopaminergic neurons in primary mesencephalic mixed neuron-glia cultures. Rat primary mixed mesencephalic neuron-glia cultures were seeded in 24-well plates at 5×105 /well and treated with 1 – 5 μg NM/ml for 10 days. Effects of NM on the dopaminergic neurons in mixed neuron-glia cultures were assessed by counting the number of dopaminergic neurons after TH staining. Results are expressed as a percentage of the vehicle-treated control cultures (mean ± s.e.m.; n = 3; * p < 0.001). (b) Microglia derived from Mac-1−/− mice exhibit less NM neurotoxicity (mean ± s.e.m.; n = 4; * p < 0.001). NM toxicity in co-cultures is inhibited in microglia derived from PHOX−/− mice (c; mean ± s.e.m.; n = 4; * p < 0.001) and by the PHOX inhibitor, apocyanin (100 μM) (d; NM 5 μg/ml; mean ± s.e.m.; n = 7; * p < 0.01).
Fig. 4. Immunostaining in the rat SN 10 days after NM injection
Rats received either phosphate buffered saline or NM at 1 μl/min for 4 min, then the total amount of NM injected into the rat SN was 3.4 μg. The needle was left in place an additional 2 min. Arrows indicate the site of injection within the SN. Representative TH immunostaining of the SN in a NM-injected rat (a), and in a vehicle-injected rat (b). Representative SN Iba-1stained microglia (c, d), GFAP stained astrocytes (e, f), and GABA neurons (g, h) from a NM-injected rat (c, e, g) and from a vehicle-injected rat (d, f, h) are also shown. Scale bar: (a – f) = 440 μm; (g, h) = 175 μm.
Similar articles
- Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson's disease.
Zecca L, Wilms H, Geick S, Claasen JH, Brandenburg LO, Holzknecht C, Panizza ML, Zucca FA, Deuschl G, Sievers J, Lucius R. Zecca L, et al. Acta Neuropathol. 2008 Jul;116(1):47-55. doi: 10.1007/s00401-008-0361-7. Epub 2008 Mar 15. Acta Neuropathol. 2008. PMID: 18343932 - HMGB1 acts on microglia Mac1 to mediate chronic neuroinflammation that drives progressive neurodegeneration.
Gao HM, Zhou H, Zhang F, Wilson BC, Kam W, Hong JS. Gao HM, et al. J Neurosci. 2011 Jan 19;31(3):1081-92. doi: 10.1523/JNEUROSCI.3732-10.2011. J Neurosci. 2011. PMID: 21248133 Free PMC article. - Protein kinase Cδ upregulation in microglia drives neuroinflammatory responses and dopaminergic neurodegeneration in experimental models of Parkinson's disease.
Gordon R, Singh N, Lawana V, Ghosh A, Harischandra DS, Jin H, Hogan C, Sarkar S, Rokad D, Panicker N, Anantharam V, Kanthasamy AG, Kanthasamy A. Gordon R, et al. Neurobiol Dis. 2016 Sep;93:96-114. doi: 10.1016/j.nbd.2016.04.008. Epub 2016 May 2. Neurobiol Dis. 2016. PMID: 27151770 Free PMC article. - Inflammation in Parkinson's diseases and other neurodegenerative diseases: cause and therapeutic implications.
Wilms H, Zecca L, Rosenstiel P, Sievers J, Deuschl G, Lucius R. Wilms H, et al. Curr Pharm Des. 2007;13(18):1925-8. doi: 10.2174/138161207780858429. Curr Pharm Des. 2007. PMID: 17584117 Review. - The role of tyrosine hydroxylase as a key player in neuromelanin synthesis and the association of neuromelanin with Parkinson's disease.
Nagatsu T, Nakashima A, Watanabe H, Ito S, Wakamatsu K, Zucca FA, Zecca L, Youdim M, Wulf M, Riederer P, Dijkstra JM. Nagatsu T, et al. J Neural Transm (Vienna). 2023 May;130(5):611-625. doi: 10.1007/s00702-023-02617-6. Epub 2023 Mar 20. J Neural Transm (Vienna). 2023. PMID: 36939908 Free PMC article. Review.
Cited by
- Neuronal vulnerability, pathogenesis, and Parkinson's disease.
Sulzer D, Surmeier DJ. Sulzer D, et al. Mov Disord. 2013 Jan;28(1):41-50. doi: 10.1002/mds.25095. Epub 2012 Jul 12. Mov Disord. 2013. PMID: 22791686 Free PMC article. Review. - Regulatory effect of bacterial melanin on the isoforms of new superoxide-producing associates from rat tissues in rotenone-induced Parkinson's disease.
Danielyan M, Nebogova K, Simonyan R, Hovsepyan A, Avetisyan Z, Simonyan K, Simonyan G, Khachatryan V, Karapetyan K. Danielyan M, et al. BMC Neurosci. 2023 Dec 20;24(1):69. doi: 10.1186/s12868-023-00838-9. BMC Neurosci. 2023. PMID: 38124101 Free PMC article. - The Efficacy of Iron Chelators for Removing Iron from Specific Brain Regions and the Pituitary-Ironing out the Brain.
Crichton RR, Ward RJ, Hider RC. Crichton RR, et al. Pharmaceuticals (Basel). 2019 Sep 17;12(3):138. doi: 10.3390/ph12030138. Pharmaceuticals (Basel). 2019. PMID: 31533229 Free PMC article. Review. - Microglial phagocytosis of live neurons.
Brown GC, Neher JJ. Brown GC, et al. Nat Rev Neurosci. 2014 Apr;15(4):209-16. doi: 10.1038/nrn3710. Nat Rev Neurosci. 2014. PMID: 24646669 Review. - Microglial Immune Response to Low Concentrations of Combustion-Generated Nanoparticles: An In Vitro Model of Brain Health.
Duffy CM, Swanson J, Northrop W, Nixon JP, Butterick TA. Duffy CM, et al. Nanomaterials (Basel). 2018 Mar 9;8(3):155. doi: 10.3390/nano8030155. Nanomaterials (Basel). 2018. PMID: 29522448 Free PMC article.
References
- Banati RB, Daniel SE, Blunt SB. Glial pathology but absence of apoptotic nigral neurons in long-standing Parkinson's disease. Mov Disord. 1998;13:221–227. - PubMed
- Berger M, Budhu S, Lu E, Li Y, Loike D, Silverstein SC, Loike JD. Different G(i)-coupled chemoattractant receptors signal qualitatively different functions in human neutrophils. J Leukoc Biol. 2002;71:798–806. - PubMed
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. - PubMed
- Chen H, Zhang SM, Hernán MA, Schwarzschild MA, Willett WC, Colditz GA, Speizer FE, Ascherio A. Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Arch Neurol. 2003;60:1059–1064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG021617/AG/NIA NIH HHS/United States
- NS38370-09/NS/NINDS NIH HHS/United States
- ZIA ES090082-14/ImNIH/Intramural NIH HHS/United States
- R21 NS062180/NS/NINDS NIH HHS/United States
- AG21617-01A1/AG/NIA NIH HHS/United States
- P01 NS011766/NS/NINDS NIH HHS/United States
- ZIA ES090082/ImNIH/Intramural NIH HHS/United States
- NS062180/NS/NINDS NIH HHS/United States
- P50 NS038370/NS/NINDS NIH HHS/United States
- NS11766-27A1/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical