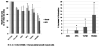Differentiation of adult stem cells into smooth muscle for vascular tissue engineering - PubMed (original) (raw)
Differentiation of adult stem cells into smooth muscle for vascular tissue engineering
Lisa J Harris et al. J Surg Res. 2011.
Abstract
Background: Herein we evaluate the potential of adipose-derived stem cells (ASC) to differentiate into smooth muscle cells (SMC) and their potential for use in a tissue-engineered vascular graft.
Materials and methods: We isolated ASC (CD13+29+90+) from the peri-umbilical adipose tissue of patients undergoing vascular surgery, and cultured them in media containing angiotensin II (AngII), sphingosylphosphorylcholine (SPC), or transforming growth factor-beta 1 (TGFβ1) for up to 3 weeks. SMC differentiation was assessed by (1) expression of early (calponin, caldesmon) and late (myosin heavy chain, MHC) SMC markers by RT-PCR, qPCR and Western blot, and (2) contraction upon plating on collagen gel. Differentiated ASCs were seeded onto a vascular graft (decellularized saphenous vein) within a bioreactor, and cell attachment was determined using confocal microscopy.
Results: Prior to differentiation, ASC expressed low levels of all three molecular markers. After culture in each differentiating medium, the extent of up-regulation of calponin, caldesmon, and MHC was variable across all cell lines. After seeding onto collagen gel, ASCs differentiated in SPC and TGFβ1 exhibit contractile properties, similar to smooth muscle cell controls. Differentiated stem cells adhered and proliferated on the vascular graft.
Conclusion: These data suggest that human adipose-derived stem cells (1) exhibit variable expression of SMC molecular markers after differentiation, (2) exhibit a contractile phenotype after differentiation with SPC and TGFβ1, and (3) proliferate on a vascular graft scaffold. Thus, ASCs are potentially useful in the construction of autologous arteries.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Figure 1. Proliferation of ASC and doubling times
Growth curve over 7 days showing ASC (n=3) cultured in non-differentiating media and with media containing growth factors ANG, SPC and TGFβ1 (error bars omitted due to juxtaposition of lines). All p-values for points on curve are not significant. Similarly, doubling times are not significantly different.
Figure 2. Expression of intermediate and late SMC markers after one week of differentiation
RT-PCR for calponin, caldesmon, and MHC was performed for 4 patient cell lines after differentiation in ANG, SPC or TGFβ1 for one week. Expression of calponin was seen with all 3 differentiating agents in patient 4 only. Up-regulation of caldesmon was seen in patients 1 and 3 only. No MHC expression was seen with any cell lines at one week.
Figure 3. Expression of intermediate and late SMC markers after differentiation for two weeks
RT-PCR for calponin, caldesmon, and MHC was performed in 6 patient cell lines after differentiation for two weeks. Up-regulation of calponin was seen in patient 3 only with TGFβ1. Caldesmon up-regulation was variable with 3 of the 6 patients showing up-regulation. Expression of MHC was seen with SPC in patient 2 and with TGFβ1 in patients 3-5. Patients 1 and 6 did not show MHC expression.
Figure 4. Quantification of SMC marker expression after two weeks of differentiation
Quantitative PCR for calponin, caldesmon, and MHC after ASC differentiation (n=3) for two weeks. Up-regulation of calponin was seen with TGFβ1. No up-regulation of caldesmon was seen by ANG, SPC, or TGFβ1. Up-regulation of MHC was seen with ANG and SPC. P-values were not statistically significant.
Figure 5. Protein expression after three weeks of differentiation
Expression of calponin, caldesmon, and MHC protein by Western blot analysis after differentiation in ANG, SPC, or TGFβ1 for three weeks. Up-regulation of calponin was seen with TGFβ1 in cell line 7 and with all three agents in cell lines 8 and 9. No up-regulation of caldesmon was seen, however caldesmon was expressed at baseline and with all three differentiating agents. No expression of MHC was seen.
Figure 6. Assessment of contraction: Collagen gel contraction assay
A. Assessment of basal contractile tone and stimulation of contraction by KCl using collagen gel lattice contraction assay (n=4). Collagen gels without cells show no evidence of contraction. Some basal contractile tone is exhibited by non-differentiated ASC and stem cells differentiated in ANG, SPC and TGFβ1. The addition of KCl results in increased contraction with ASC differentiated with SPC and TGFβ1, but not ANG when compared to undifferentiated ASC. Human arterial smooth muscle cells were used as a positive control. B. Percent increase in contraction after stimulation with KCl normalized to undifferentiated ASC. Stem cells differentiated in SPC and TGFβ1 show significantly increased contraction (p < 0.05) similar to human arterial SMCs.
Figure 7. Attachment of differentiated ASC to vascular graft scaffold
ASCs differentiated in SPC for two weeks were seeded onto vascular graft scaffold (decellularized human greater saphenous vein) (A) and cultured in bioreactor system (B) for 24 hours. Cell attachment as viewed by confocal microscopy at 40× magnification (C). Similar results were obtained with cells differentiated in ANG and TGFβ1. Vascular graft scaffold as viewed by confocal microscopy prior to seeding (D).
Similar articles
- Complete Myogenic Differentiation of Adipogenic Stem Cells Requires Both Biochemical and Mechanical Stimulation.
Helms F, Lau S, Klingenberg M, Aper T, Haverich A, Wilhelmi M, Böer U. Helms F, et al. Ann Biomed Eng. 2020 Mar;48(3):913-926. doi: 10.1007/s10439-019-02234-z. Epub 2019 Feb 27. Ann Biomed Eng. 2020. PMID: 30815762 - Differentiation of adipose-derived stem cells into contractile smooth muscle cells induced by transforming growth factor-beta1 and bone morphogenetic protein-4.
Wang C, Yin S, Cen L, Liu Q, Liu W, Cao Y, Cui L. Wang C, et al. Tissue Eng Part A. 2010 Apr;16(4):1201-13. doi: 10.1089/ten.TEA.2009.0303. Tissue Eng Part A. 2010. PMID: 19895205 - Sphingosylphosphorylcholine induces differentiation of human mesenchymal stem cells into smooth-muscle-like cells through a TGF-beta-dependent mechanism.
Jeon ES, Moon HJ, Lee MJ, Song HY, Kim YM, Bae YC, Jung JS, Kim JH. Jeon ES, et al. J Cell Sci. 2006 Dec 1;119(Pt 23):4994-5005. doi: 10.1242/jcs.03281. Epub 2006 Nov 14. J Cell Sci. 2006. PMID: 17105765 - Modulation of Smooth Muscle Cell Phenotype for Translation of Tissue-Engineered Vascular Grafts.
Pineda-Castillo SA, Acar H, Detamore MS, Holzapfel GA, Lee CH. Pineda-Castillo SA, et al. Tissue Eng Part B Rev. 2023 Oct;29(5):574-588. doi: 10.1089/ten.TEB.2023.0006. Epub 2023 May 30. Tissue Eng Part B Rev. 2023. PMID: 37166394 Free PMC article. Review.
Cited by
- Whole Organ Tissue Vascularization: Engineering the Tree to Develop the Fruits.
Pellegata AF, Tedeschi AM, De Coppi P. Pellegata AF, et al. Front Bioeng Biotechnol. 2018 May 14;6:56. doi: 10.3389/fbioe.2018.00056. eCollection 2018. Front Bioeng Biotechnol. 2018. PMID: 29868573 Free PMC article. Review. - A novel method for differentiation of human mesenchymal stem cells into smooth muscle-like cells on clinically deliverable thermally induced phase separation microspheres.
Parmar N, Ahmadi R, Day RM. Parmar N, et al. Tissue Eng Part C Methods. 2015 Apr;21(4):404-12. doi: 10.1089/ten.TEC.2014.0431. Epub 2014 Oct 14. Tissue Eng Part C Methods. 2015. PMID: 25205072 Free PMC article. - Emerging Strategies in Mesenchymal Stem Cell-Based Cardiovascular Therapeutics.
Kumar R, Mishra N, Tran T, Kumar M, Vijayaraghavalu S, Gurusamy N. Kumar R, et al. Cells. 2024 May 17;13(10):855. doi: 10.3390/cells13100855. Cells. 2024. PMID: 38786076 Free PMC article. Review. - Superparamagnetic iron oxide nanoparticles regulate smooth muscle cell phenotype.
Angelopoulos I, Southern P, Pankhurst QA, Day RM. Angelopoulos I, et al. J Biomed Mater Res A. 2016 Oct;104(10):2412-9. doi: 10.1002/jbm.a.35780. Epub 2016 May 30. J Biomed Mater Res A. 2016. PMID: 27176658 Free PMC article. - Regenerative therapy after cancer: what are the risks?
Donnenberg VS, Zimmerlin L, Rubin JP, Donnenberg AD. Donnenberg VS, et al. Tissue Eng Part B Rev. 2010 Dec;16(6):567-75. doi: 10.1089/ten.TEB.2010.0352. Epub 2010 Nov 2. Tissue Eng Part B Rev. 2010. PMID: 20726819 Free PMC article. Review.
References
- Veith FL, Moss CM, Sprayregen S, Montefusco C. Pre-operative saphenous venography in arterial reconstructive surgery of the lower extremity. Surgery. 1979;85:253. - PubMed
- Bergan JJ, Veith FJ, Bernhard VM, et al. Randomization of autogenous vein and polytetrafluoroethylene grafts in femoral-distal reconstruction. Surgery. 1982;92:921. - PubMed
- Veith FJ, Gupta SK, Ascer E, et al. Six year prospective multicenter randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3:104. - PubMed
- Riha GM, Lin PH, Lumsden AB, et al. Application of stem cells for vascular tissue engineering. Tissue Eng. 2005;11:1535. - PubMed
- Barrilleaux B, Phinney DG, Prockop Darwin, O'Connor KC. Review: Ex vivo engineering of living tissues with adult stem cells. Tissue Eng. 2006;12:1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous