Macrophage death and defective inflammation resolution in atherosclerosis - PubMed (original) (raw)
Review
Macrophage death and defective inflammation resolution in atherosclerosis
Ira Tabas. Nat Rev Immunol. 2010 Jan.
Abstract
A key event in atherosclerosis is a maladaptive inflammatory response to subendothelial lipoproteins. A crucial aspect of this response is a failure to resolve inflammation, which normally involves the suppression of inflammatory cell influx, effective clearance of apoptotic cells and promotion of inflammatory cell egress. Defects in these processes promote the progression of atherosclerotic lesions into dangerous plaques, which can trigger atherothrombotic vascular disease, the leading cause of death in industrialized societies. In this Review I provide an overview of these concepts, with a focus on macrophage death and defective apoptotic cell clearance, and discuss new therapeutic strategies designed to boost inflammation resolution in atherosclerosis.
Figures
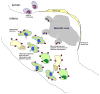
Figure 1. Schematic of a dangerous, or “vulnerable,” atherosclerotic plaque showing the hallmarks of defective resolution of inflammation
Inflammatory cells, including lipid-laden macrophage (Mφ) foam cells, have accumulated in the intima, resulting from both persistent influx of new cells, particularly monocytes, and defective egress of the resident cells. Moreover, dead macrophages are not efficiently cleared by the process of efferocytosis, and so they undergo post- apoptotic necrosis. This process contributes to the formation of the necrotic core, which contributes to plaque disruption, particularly thinning of the fibrous cap. If the process continues, there will be a breach in the fibrous cap, leading to lumenal thrombosis and arterial occlusion.
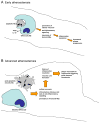
Figure 2. Efferocytosis and inflammation resolution in early and advanced atherosclerosis
A In early atherosclerotic lesions, efferocytosis is efficient, leading to rapid clearing of apoptotic macrophages (Mφs). This process prevents post-apoptotic cellular necrosis, elicits the production of anti-inflammatory cytokines, and clears macrophages from the lesions. The result of this inflammation resolution process is decreased plaque progression. B In advanced lesions, efferocytes do not function properly and this apoptotic macrophages become secondarily necrotic. The necrotic material is a stimulus for inflammation, and the normal anti-inflammatory signaling associated with efferocytosis does not occur. Moreover, an important mean for ridding the lesion of inflammatory macrophages is lost. Thus, inflammation resolution fails to occur normally, and necrotic macrophages coalesce into necrotic cores. These features define plaques that are vulnerable to rupture. which in turn can trigger acute lumenal thrombosis and arterial occlusion.
Similar articles
- Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency.
Tabas I. Tabas I. Arterioscler Thromb Vasc Biol. 2005 Nov;25(11):2255-64. doi: 10.1161/01.ATV.0000184783.04864.9f. Epub 2005 Sep 1. Arterioscler Thromb Vasc Biol. 2005. PMID: 16141399 Review. - 2016 Russell Ross Memorial Lecture in Vascular Biology: Molecular-Cellular Mechanisms in the Progression of Atherosclerosis.
Tabas I. Tabas I. Arterioscler Thromb Vasc Biol. 2017 Feb;37(2):183-189. doi: 10.1161/ATVBAHA.116.308036. Epub 2016 Dec 15. Arterioscler Thromb Vasc Biol. 2017. PMID: 27979856 Free PMC article. Review. - Atherosclerosis - A matter of unresolved inflammation.
Viola J, Soehnlein O. Viola J, et al. Semin Immunol. 2015 May;27(3):184-93. doi: 10.1016/j.smim.2015.03.013. Epub 2015 Apr 10. Semin Immunol. 2015. PMID: 25865626 Review. - Dead cell and debris clearance in the atherosclerotic plaque: Mechanisms and therapeutic opportunities to promote inflammation resolution.
Dhawan UK, Singhal A, Subramanian M. Dhawan UK, et al. Pharmacol Res. 2021 Aug;170:105699. doi: 10.1016/j.phrs.2021.105699. Epub 2021 Jun 2. Pharmacol Res. 2021. PMID: 34087352 Review. - Apoptotic cell clearance: basic biology and therapeutic potential.
Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Poon IK, et al. Nat Rev Immunol. 2014 Mar;14(3):166-80. doi: 10.1038/nri3607. Epub 2014 Jan 31. Nat Rev Immunol. 2014. PMID: 24481336 Free PMC article. Review.
Cited by
- Immune and inflammatory mechanisms of abdominal aortic aneurysm.
Márquez-Sánchez AC, Koltsova EK. Márquez-Sánchez AC, et al. Front Immunol. 2022 Oct 5;13:989933. doi: 10.3389/fimmu.2022.989933. eCollection 2022. Front Immunol. 2022. PMID: 36275758 Free PMC article. Review. - Upconversion nanoparticle-mediated photodynamic therapy induces THP-1 macrophage apoptosis via ROS bursts and activation of the mitochondrial caspase pathway.
Zhu X, Wang H, Zheng L, Zhong Z, Li X, Zhao J, Kou J, Jiang Y, Zheng X, Liu Z, Li H, Cao W, Tian Y, Wang Y, Yang L. Zhu X, et al. Int J Nanomedicine. 2015 May 22;10:3719-36. doi: 10.2147/IJN.S82162. eCollection 2015. Int J Nanomedicine. 2015. PMID: 26045663 Free PMC article. - Involvement of calmodulin and calmodulin kinase II in tumor necrosis factor alpha-induced survival of bone marrow derived macrophages.
Tano JY, Lee RH, Vazquez G. Tano JY, et al. Biochem Biophys Res Commun. 2012 Oct 12;427(1):178-84. doi: 10.1016/j.bbrc.2012.09.038. Epub 2012 Sep 16. Biochem Biophys Res Commun. 2012. PMID: 22989752 Free PMC article. - Inflammation in atherosclerosis.
Libby P. Libby P. Arterioscler Thromb Vasc Biol. 2012 Sep;32(9):2045-51. doi: 10.1161/ATVBAHA.108.179705. Arterioscler Thromb Vasc Biol. 2012. PMID: 22895665 Free PMC article. Review. - Recent progress of endoplasmic reticulum stress in the mechanism of atherosclerosis.
Ni L, Yang L, Lin Y. Ni L, et al. Front Cardiovasc Med. 2024 Jul 12;11:1413441. doi: 10.3389/fcvm.2024.1413441. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39070554 Free PMC article. Review.
References
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. - PubMed
- Braunwald E. Cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. - PubMed
- NHLBI Morbidity and Mortality Chart Book. (2004).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical