Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen - PubMed (original) (raw)
Case Reports
doi: 10.1016/S1474-4422(09)70324-2. Epub 2009 Dec 2.
Meizan Lai, Xiaoyu Peng, Ethan Hughes, Radu Constantinescu, Jeffrey Raizer, Daniel Friedman, Mark B Skeen, Wolfgang Grisold, Akio Kimura, Kouichi Ohta, Takahiro Iizuka, Miguel Guzman, Francesc Graus, Stephen J Moss, Rita Balice-Gordon, Josep Dalmau
Affiliations
- PMID: 19962348
- PMCID: PMC2824142
- DOI: 10.1016/S1474-4422(09)70324-2
Case Reports
Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterisation of the antigen
Eric Lancaster et al. Lancet Neurol. 2010 Jan.
Abstract
Background: Some encephalitides or seizure disorders once thought idiopathic now seem to be immune mediated. We aimed to describe the clinical features of one such disorder and to identify the autoantigen involved.
Methods: 15 patients who were suspected to have paraneoplastic or immune-mediated limbic encephalitis were clinically assessed. Confocal microscopy, immunoprecipitation, and mass spectrometry were used to characterise the autoantigen. An assay of HEK293 cells transfected with rodent GABA(B1) or GABA(B2) receptor subunits was used as a serological test. 91 patients with encephalitis suspected to be paraneoplastic or immune mediated and 13 individuals with syndromes associated with antibodies to glutamic acid decarboxylase 65 were used as controls.
Findings: All patients presented with early or prominent seizures; other symptoms, MRI, and electroencephalography findings were consistent with predominant limbic dysfunction. All patients had antibodies (mainly IgG1) against a neuronal cell-surface antigen; in three patients antibodies were detected only in CSF. Immunoprecipitation and mass spectrometry showed that the antibodies recognise the B1 subunit of the GABA(B) receptor, an inhibitory receptor that has been associated with seizures and memory dysfunction when disrupted. Confocal microscopy showed colocalisation of the antibody with GABA(B) receptors. Seven of 15 patients had tumours, five of which were small-cell lung cancer, and seven patients had non-neuronal autoantibodies. Although nine of ten patients who received immunotherapy and cancer treatment (when a tumour was found) showed neurological improvement, none of the four patients who were not similarly treated improved (p=0.005). Low levels of GABA(B1) receptor antibodies were identified in two of 104 controls (p<0.0001).
Interpretation: GABA(B) receptor autoimmune encephalitis is a potentially treatable disorder characterised by seizures and, in some patients, associated with small-cell lung cancer and with other autoantibodies.
Funding: National Institutes of Health.
Copyright 2010 Elsevier Ltd. All rights reserved.
Figures
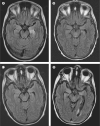
Figure 1. MRI of a patient with GABAB receptor antibodies and limbic encephalitis
Axial fluid-attenuated inversion recovery (FLAIR) MRI from patient 1 at presentation (A) showed increased signal in the medial temporal lobes, which was more pronounced on the left. Repeat study at 1 month (B) showed improvement of the FLAIR signal that remained stable at 3 months and 9 months (C, D), with development of mild generalised atrophy (the patient received standard whole-brain radiation therapy as prophylaxis for small-cell lung cancer metastases).
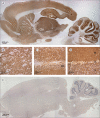
Figure 2. Immunolabelling of rat brain with patients' antibodies
Sagittal section of rat brain immunolabelled with CSF of a patient with limbic encephalitis (A) and a control individual (E). Note the extensive staining in A of the neuropil of thalamus (B), hippocampus (C), cerebellum (D), and cerebral cortex. DG=dentate gyrus. M=molecular layer. P=Purkinje cell layer. G=granular cell layer. Avidin–biotin–peroxidase method; sections counterstained with haematoxylin.
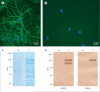
Figure 3. Culture of rat hippocampal neurons incubated (live, non-permeabilised) with the CSF of a patient with limbic encephalitis and a control individual
Note the intense punctate reactivity of patient's antibodies with cell surface antigens (A) and the absence of reactivity in the control (B); nuclei of neurons stained with 4',6-diamidino-2-phenylindole (DAPI). The surface antigens were precipitated using the antibodies within the patient's serum, and then electrophoretically separated and visualised with EZBlue (C). Patient's antibodies (P) precipitated two main protein bands at about 105 kDa and 90 kDa; these bands are not seen in the precipitate using serum from a control individual (N). Sequencing of the 105 kDa band by use of mass spectrometry showed it contained the B1 and B2 subunits of the GABAB receptor (webappendix). The 90 kDa and other smaller bands were proteolytic fragments and patient's IgG products. Subsequent transfer of the gel to nitrocellulose and immunoblotting with antibodies specific for each of the GABAB (D) subunits confirmed that patient's antibodies precipitated the B1 and B2 subunits (105 kDa) and that the 90 kDa band was a proteolytic fragment of B1.
Figure 4. Confocal image of a cultured triple labelled embryonic rat hippocampal neuron
Patient's antibodies are in green, a guineapig polyclonal antibody against an intracellular epitope of the GABAB1 receptor is in red, and an antibody to the presynaptic marker Bassoon is in blue (A). Area of dendrite from the same neuron showing patient's antibody staining (B), guineapig polyclonal GABAB1 receptor antibody staining (C), both patient and guineapig antibody staining (D), and triple staining (E). The colocalisation of labelling of the dendrites of 23 neurons was quantified (F). This suggests that patients' antibodies bind both synaptic and extrasynaptic GABAB receptors.
Figure 5. Detection of antibodies to the GABAB1 subunit using a HEK293 cell-based assay
HEK293 cells transfected with the GABAB1 receptor subunit show reactivity with CSF from a patient with limbic encephalitis (A) and a polyclonal antibody against the B1 subunit of the GABAB receptor (B); both reactivities are merged in C. Similarly transfected cells do not react with CSF from a control individual (D) but do show reactivity with a polyclonal antibody against the B1 subunit of the GABAB receptor (E); reactivities merged in F. Immunofluorescent method.
Comment in
- Autoimmune limbic encephalitis: an expanding concept.
Honnorat J. Honnorat J. Lancet Neurol. 2010 Jan;9(1):24-5. doi: 10.1016/S1474-4422(09)70333-3. Epub 2009 Dec 2. Lancet Neurol. 2010. PMID: 19962349 No abstract available. - Complement-mediated cytotoxicity of antibodies to the GABA(B) receptor.
Batocchi AP, Della Marca G, Frisullo G. Batocchi AP, et al. Lancet Neurol. 2010 Apr;9(4):343; author reply 343-4. doi: 10.1016/S1474-4422(10)70070-3. Lancet Neurol. 2010. PMID: 20298957 No abstract available.
Similar articles
- Complement-mediated cytotoxicity of antibodies to the GABA(B) receptor.
Batocchi AP, Della Marca G, Frisullo G. Batocchi AP, et al. Lancet Neurol. 2010 Apr;9(4):343; author reply 343-4. doi: 10.1016/S1474-4422(10)70070-3. Lancet Neurol. 2010. PMID: 20298957 No abstract available. - Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies.
Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason WP, Kruer MC, Ritacco DG, Grisold W, Meaney BF, Alcalá C, Sillevis-Smitt P, Titulaer MJ, Balice-Gordon R, Graus F, Dalmau J. Petit-Pedrol M, et al. Lancet Neurol. 2014 Mar;13(3):276-86. doi: 10.1016/S1474-4422(13)70299-0. Epub 2014 Jan 22. Lancet Neurol. 2014. PMID: 24462240 Free PMC article. - Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients.
Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Gultekin SH, et al. Brain. 2000 Jul;123 ( Pt 7):1481-94. doi: 10.1093/brain/123.7.1481. Brain. 2000. PMID: 10869059 Clinical Trial. - Glutamate receptor antibodies in neurological diseases: anti-AMPA-GluR3 antibodies, anti-NMDA-NR1 antibodies, anti-NMDA-NR2A/B antibodies, anti-mGluR1 antibodies or anti-mGluR5 antibodies are present in subpopulations of patients with either: epilepsy, encephalitis, cerebellar ataxia, systemic lupus erythematosus (SLE) and neuropsychiatric SLE, Sjogren's syndrome, schizophrenia, mania or stroke. These autoimmune anti-glutamate receptor antibodies can bind neurons in few brain regions, activate glutamate receptors, decrease glutamate receptor's expression, impair glutamate-induced signaling and function, activate blood brain barrier endothelial cells, kill neurons, damage the brain, induce behavioral/psychiatric/cognitive abnormalities and ataxia in animal models, and can be removed or silenced in some patients by immunotherapy.
Levite M. Levite M. J Neural Transm (Vienna). 2014 Aug;121(8):1029-75. doi: 10.1007/s00702-014-1193-3. Epub 2014 Aug 1. J Neural Transm (Vienna). 2014. PMID: 25081016 Review. - Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients.
Höftberger R, Titulaer MJ, Sabater L, Dome B, Rózsás A, Hegedus B, Hoda MA, Laszlo V, Ankersmit HJ, Harms L, Boyero S, de Felipe A, Saiz A, Dalmau J, Graus F. Höftberger R, et al. Neurology. 2013 Oct 22;81(17):1500-6. doi: 10.1212/WNL.0b013e3182a9585f. Epub 2013 Sep 25. Neurology. 2013. PMID: 24068784 Free PMC article. Review.
Cited by
- Autoimmune encephalopathies presenting as dementia of subacute onset and rapid progression.
Banks SA, Sechi E, Flanagan EP. Banks SA, et al. Ther Adv Neurol Disord. 2021 Mar 19;14:1756286421998906. doi: 10.1177/1756286421998906. eCollection 2021. Ther Adv Neurol Disord. 2021. PMID: 33796145 Free PMC article. Review. - Regulation of neuronal GABA(B) receptor functions by subunit composition.
Gassmann M, Bettler B. Gassmann M, et al. Nat Rev Neurosci. 2012 May 18;13(6):380-94. doi: 10.1038/nrn3249. Nat Rev Neurosci. 2012. PMID: 22595784 Review. - Postvaccinal GABA-B receptor antibody encephalitis after ChAdOx1 nCoV-19 vaccination.
Ahn SJ, Lee ST, Chu K. Ahn SJ, et al. Ann Clin Transl Neurol. 2022 Oct;9(10):1673-1678. doi: 10.1002/acn3.51659. Epub 2022 Sep 2. Ann Clin Transl Neurol. 2022. PMID: 36053935 Free PMC article. - The GABAB Receptor-Structure, Ligand Binding and Drug Development.
Evenseth LSM, Gabrielsen M, Sylte I. Evenseth LSM, et al. Molecules. 2020 Jul 7;25(13):3093. doi: 10.3390/molecules25133093. Molecules. 2020. PMID: 32646032 Free PMC article. Review. - An Australian State-Based Cohort Study of Autoimmune Encephalitis Cases Detailing Clinical Presentation, Investigation Results, and Response to Therapy.
Swayne A, Warren N, Prain K, Gillis D, O'Gorman C, Tsang BK, Muller C, Broadley S, Adam RJ, McCombe P, Wong RC, Blum S. Swayne A, et al. Front Neurol. 2021 Feb 22;12:607773. doi: 10.3389/fneur.2021.607773. eCollection 2021. Front Neurol. 2021. PMID: 33692738 Free PMC article.
References
- Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci. 2004;5:952–62. - PubMed
- Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–91. - PubMed
- Schuler V, Luscher C, Blanchet C, et al. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABA(B) responses in mice lacking GABA(B(1)) Neuron. 2001;31:47–58. - PubMed
- Prosser HM, Gill CH, Hirst WD, et al. Epileptogenesis and enhanced prepulse inhibition in GABA(B1)-deficient mice. Mol Cell Neurosci. 2001;17:1059–70. - PubMed
- Lau CG, Zukin RS. NMDA receptor trafficking in synaptic plasticity and neuropsychiatric disorders. Nat Rev Neurosci. 2007;8:413–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS056359/NS/NINDS NIH HHS/United States
- R01 NS048045/NS/NINDS NIH HHS/United States
- NS048045/NS/NINDS NIH HHS/United States
- NS051195/NS/NINDS NIH HHS/United States
- R01CA089054/CA/NCI NIH HHS/United States
- R01 NS046478/NS/NINDS NIH HHS/United States
- R56 MH057683/MH/NIMH NIH HHS/United States
- R01 CA107192-04/CA/NCI NIH HHS/United States
- R21 MH057683/MH/NIMH NIH HHS/United States
- R01CA107192/CA/NCI NIH HHS/United States
- R01 NS056359/NS/NINDS NIH HHS/United States
- P01NS054900/NS/NINDS NIH HHS/United States
- R01 CA089054/CA/NCI NIH HHS/United States
- R01 NS051195/NS/NINDS NIH HHS/United States
- R01 CA089054-06A2/CA/NCI NIH HHS/United States
- R01 MH057683/MH/NIMH NIH HHS/United States
- P01 NS054900/NS/NINDS NIH HHS/United States
- NS046478/NS/NINDS NIH HHS/United States
- R01 CA107192/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical