TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress - PubMed (original) (raw)
TRPV4-mediated endothelial Ca2+ influx and vasodilation in response to shear stress
Suelhem A Mendoza et al. Am J Physiol Heart Circ Physiol. 2010 Feb.
Abstract
The transient receptor potential vallinoid type 4 (TRPV4) channel has been implicated in the endothelial shear response and flow-mediated dilation, although the precise functions of this channel remain poorly understood. In the present study, we investigated the role of TRPV4 in shear stress-induced endothelial Ca(2+) entry and the potential link between this signaling response and relaxation of small resistance arteries. Using immunohistochemical analysis and RT-PCR, we detected strong expression of TRPV4 protein and mRNA in the endothelium in situ and endothelial cells freshly isolated from mouse small mesenteric arteries. The selective TRPV4 agonist GSK1016790A increased endothelial Ca(2+) and induced potent relaxation of small mesenteric arteries from wild-type (WT) but not TRPV4(-/-) mice. Luminal flow elicited endothelium-dependent relaxations that involved both nitric oxide and EDHFs. Both nitric oxide and EDHF components of flow-mediated relaxation were markedly reduced in TRPV4(-/-) mice compared with WT controls. Using a fura-2/Mn(2+) quenching assay, shear was observed to produce rapid Ca(2+) influx in endothelial cells, which was markedly inhibited by the TRPV4 channel blocker ruthenium red and TRPV4-specific short interfering RNA. Flow elicited a similar TRPV4-mediated Ca(2+) entry in HEK-293 cells transfected with TRPV4 channels but not in nontransfected cells. Collectively, these data indicate that TRPV4 may be a potential candidate of mechanosensitive channels in endothelial cells through which the shear stimulus is transduced into Ca(2+) signaling, leading to the release of endothelial relaxing factors and flow-mediated dilation of small resistance arteries.
Figures
Fig. 1.
Transient receptor potential vanilloid 4 (TRPV4) expression in endothelial cells (ECs) of mouse mesenteric arteries. A, top: hematoxylin and eosin staining of wild-type (WT) and TRPV4 knockout (TRPV4−/−) mesenteric arteries. Bottom, immunohistochemical staining of TRPV4 was seen in the endothelium of mesenteric arteries from WT but not TRPV4−/− mice. Scale bar = 5 μm. B: RT-PCR analysis indicated that TRPV4 mRNA was expressed in ECs but not smooth muscle cells (SMCs) freshly isolated from mesenteric arteries of WT animals. The same samples were amplified for the expression of PECAM-1, an endothelial marker, and β-actin, a housekeeping gene present in both ECs and SMCs. M, DNA marker. Results are representative of 3 independent experiments.
Fig. 2.
TRPV4-mediated endothelial Ca2+ responses and vasodilation in mouse mesenteric arteries. A: addition of the TRPV4-specific agonist GSK1016790A (GSK; 100 nM) caused a rapid increase in the intracellular Ca2+ concentration ([Ca2+]i) in WT, which was blocked by ruthenium red (RuR; 10 μM), a nonselective TRPV4 channel blocker. GSK had no effect in TRPV4−/− mice. Top, representative traces; bottom, summarized data. n = 3–5 mice (20–30 ECs/vessel). *P < 0.05 vs. basal; #P < 0.05 vs. GSK. B: GSK (100 nM) induced potent endothelium-dependent relaxation of mesenteric arteries in WT but not TRPV4−/− animals. This dilation was largely abolished by RuR (10 μM) given before or after GSK. n = 3–6 mice. *P < 0.05 vs. GSK. C: GSK-induced vasodilation was reduced by _N_G-nitro-
l
-arginine methyl ester (
l
-NAME; 100 μM) and abolished by
l
-NAME plus high K+. n = 3 mice. *P < 0.05 vs. control.
Fig. 3.
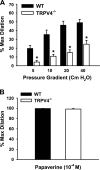
Role of TRPV4 in flow-induced dilation of mouse mesenteric arteries. A: mesenteric arteries were cannulated and pressurized, and fluid flow was generated by an increasing pressure gradient ranging from 5 to 40 cmH2O. Flow-induced dilations were reduced in TRPV4−/− compared with WT mice. n = 6 mice. *P < 0.05 vs. WT. B: endothelium-independent relaxations in response to papaverine (100 μM) were similar between WT and TRPV4−/− mice. n = 6 mice.
Fig. 4.
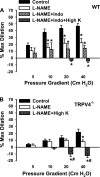
Effects of
l
-NAME, indomethacin (Indo), and high K+ on flow-induced dilation of mouse mesenteric arteries. A: in WT animals,
l
-NAME (100 μM) inhibited flow-mediated dilations, and the addition of Indo had no further effect. The relaxations were blocked by
l
-NAME plus Indo and high K+. n = 6 mice. *P < 0.05 vs. control. B: in TRPV4−/− mice, flow-induced dilations were inhibited by
l
-NAME and converted to constrictions by
l
-NAME plus high K+. n = 6 mice. *P < 0.05 vs. control. #P < 0.05 vs.
l
-NAME.
Fig. 5.
Role of TRPV4 in flow-induced Ca2+ entry in ECs. Human coronary artery ECs (HCAECs) were loaded with fura-2, and the Mn2+ quenching technique was used to measure Ca2+ influx [fluorescence at 360-nm excitation (F360); solid line] along with Ca2+ release [ratio of fluorescence at 340- to 380-nm excitation (F340/F380); dotted line]. GSK (5 nM) induced marked Ca2+ influx (A), and this response was inhibited by RuR (2 μM; B). In contrast, bradykinin (BK; 1 μM) elicited a rapid Ca2+ release that was accompanied with smaller Ca2+ influx. Similar to GSK, fluid flow (10 dyn/cm2) induced rapid Ca2+ influx (C), which was inhibited by the TRPV4 blocker RuR (D). E: baseline Ca2+ influx. F: summarized data. n = 3–6 independent experiments (20–30 ECs/assay). *P < 0.05 vs. basal; #P < 0.05 vs. GSK or flow.
Fig. 6.
Effect of TRPV4 short interfering (si)RNA on flow-induced Ca2+ entry in ECs. A: RT-PCR analysis of TRPV4 mRNA levels in HCAECs treated with nontargeting siControl (100 nM) or TRPV4-specific siRNA (50–100 nM). B: Western blot analysis of TRPV4 protein expression in HCAECs treated with siControl (100 nM) or TRPV4 siRNA (100 nM). PECAM-1 and β-actin were used as loading controls for RT-PCR and Western blot analysis, respectively. Data are representative of 3–4 independent experiments. C: TRPV4 siRNA reduced cytosolic Ca2+ increases in response to the TRPV4 agonist GSK (5 nM) in fura-2-loaded HCAECs. n = 6. *P < 0.05 vs. siControl. D: TRPV4 siRNA inhibited flow (10 dyn/cm2)-induced Ca2+ influx in HCAECs as indicated by the fura-2/Mn2+ quenching assay. Data are presented as changes of the slope of fura-2/Mn2+ quenching over baseline; n = 4. *P < 0.05 vs. siControl.
Fig. 7.
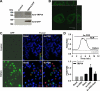
Expression of functional TRPV4 channels in human embryonice kidney (HEK)-293 cells. Cells were transiently transfected with a mammalian expression vector encoding human TRPV4 with a COOH-terminal green fluorescent protein (GFP) tag. Expression of TRPV4-GFP fusion proteins was confirmed by Western blot analysis with antibodies against both TRPV4 and GFP (A) and by confocal fluorescence microscopy (B). In transfected cells, the TRPV4 agonist 4α-phorbol-12,13-didecanoate (4α-PDD; 5 μM) induced a marked increase in [Ca2+]i, whereas 4α-PDD had no significant effect in nontransfected cells (C). D: representative traces of Ca2+ responses showing that the 4α-PDD-induced Ca2+ increase was subsequently reversed by RuR (2 μM) in cells expressing TRPV4-GFP fusion proteins (top). Bottom, summarized data of Ca2+ responses to 4α-PDD. n = 3 independent experiments (20–30 cells/assay). *P < 0.05 vs. basal; #P < 0.05 vs. 4α-PDD.
Fig. 8.
Flow-induced Ca2+ response in HEK-293 cells expressing TRPV4 channels. A: fluid flow (10 dyn/cm2) elicited a rapid increase in [Ca2+]i in TRPV4-transfected cells but not in nontransfected controls. B, left: representative traces of Ca2+ responses to flow. Right, summarized data. n = 6 independent experiments (20–30 cells/assay). *P < 0.05 vs. basal. C: compared with nontransfected cells, HEK-293 cells expressing TRPV4 channels exhibited a marked increase in Ca2+ influx in response to flow. This increase was blocked by RuR (2 μM). n = 3–5 independent experiments (20–30 cells/assay). *P < 0.05 vs. control; #P < 0.05 vs. flow.
Similar articles
- Transient receptor potential vanilloid type 4-deficient mice exhibit impaired endothelium-dependent relaxation induced by acetylcholine in vitro and in vivo.
Zhang DX, Mendoza SA, Bubolz AH, Mizuno A, Ge ZD, Li R, Warltier DC, Suzuki M, Gutterman DD. Zhang DX, et al. Hypertension. 2009 Mar;53(3):532-8. doi: 10.1161/HYPERTENSIONAHA.108.127100. Epub 2009 Feb 2. Hypertension. 2009. PMID: 19188524 Free PMC article. - Heteromeric TRPV4/TRPC1 channels mediate calcium-sensing receptor-induced relaxations and nitric oxide production in mesenteric arteries: comparative study using wild-type and TRPC1-/- mice.
Greenberg HZE, Carlton-Carew SRE, Zargaran AK, Jahan KS, Birnbaumer L, Albert AP. Greenberg HZE, et al. Channels (Austin). 2019 Dec;13(1):410-423. doi: 10.1080/19336950.2019.1673131. Epub 2019 Oct 11. Channels (Austin). 2019. PMID: 31603369 Free PMC article. - Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation.
Köhler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Köhler R, et al. Arterioscler Thromb Vasc Biol. 2006 Jul;26(7):1495-502. doi: 10.1161/01.ATV.0000225698.36212.6a. Epub 2006 May 4. Arterioscler Thromb Vasc Biol. 2006. PMID: 16675722 - Role of TRPV4 channel in vasodilation and neovascularization.
Chen M, Li X. Chen M, et al. Microcirculation. 2021 Aug;28(6):e12703. doi: 10.1111/micc.12703. Epub 2021 May 24. Microcirculation. 2021. PMID: 33971061 Review. - TRPV4 and the regulation of vascular tone.
Filosa JA, Yao X, Rath G. Filosa JA, et al. J Cardiovasc Pharmacol. 2013 Feb;61(2):113-9. doi: 10.1097/FJC.0b013e318279ba42. J Cardiovasc Pharmacol. 2013. PMID: 23107877 Free PMC article. Review.
Cited by
- The role of TRPV4 channels in ocular function and pathologies.
Guarino BD, Paruchuri S, Thodeti CK. Guarino BD, et al. Exp Eye Res. 2020 Dec;201:108257. doi: 10.1016/j.exer.2020.108257. Epub 2020 Sep 29. Exp Eye Res. 2020. PMID: 32979394 Free PMC article. Review. - Roles of TRPV4 in Regulating Circulating Angiogenic Cells to Promote Coronary Microvascular Regeneration.
Yang W, Wang H, Guo Q, Xu X, Guo T, Sun L. Yang W, et al. J Cardiovasc Transl Res. 2023 Apr;16(2):414-426. doi: 10.1007/s12265-022-10305-1. Epub 2022 Sep 14. J Cardiovasc Transl Res. 2023. PMID: 36103035 - Endothelial control of vasodilation: integration of myoendothelial microdomain signalling and modulation by epoxyeicosatrienoic acids.
Ellinsworth DC, Earley S, Murphy TV, Sandow SL. Ellinsworth DC, et al. Pflugers Arch. 2014 Mar;466(3):389-405. doi: 10.1007/s00424-013-1303-3. Epub 2013 Jun 8. Pflugers Arch. 2014. PMID: 23748495 Free PMC article. Review. - Hydrogen sulfide-induced vasodilation mediated by endothelial TRPV4 channels.
Naik JS, Osmond JM, Walker BR, Kanagy NL. Naik JS, et al. Am J Physiol Heart Circ Physiol. 2016 Dec 1;311(6):H1437-H1444. doi: 10.1152/ajpheart.00465.2016. Epub 2016 Oct 7. Am J Physiol Heart Circ Physiol. 2016. PMID: 27765747 Free PMC article. - Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force.
Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Loukin S, et al. J Biol Chem. 2010 Aug 27;285(35):27176-27181. doi: 10.1074/jbc.M110.143370. Epub 2010 Jul 6. J Biol Chem. 2010. PMID: 20605796 Free PMC article.
References
- Ayajiki K, Kindermann M, Hecker M, Fleming I, Busse R. Intracellular pH and tyrosine phosphorylation but not calcium determine shear stress induced nitric oxide production in native endothelial cells. Circ Res 78: 750–758, 1996 - PubMed
- Basset O, Boittin FX, Dorchies OM, Chatton JY, van Breemen C, Ruegg UT. Involvement of inositol 1,4,5-trisphosphate in nicotinic calcium responses in dystrophic myotubes assessed by near-plasma membrane calcium measurement. J Biol Chem 279: 47092–47100, 2004 - PubMed
- Bratz IN, Dick GM, Tune JD, Edwards JM, Neeb ZP, Dincer UD, Sturek M. Impaired capsaicin-induced relaxation of coronary arteries in a porcine model of the metabolic syndrome. Am J Physiol Heart Circ Physiol 294: H2489–H2496, 2008 - PubMed
- Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension 17: 187–193, 1991 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL68138/HL/NHLBI NIH HHS/United States
- R01-HL080704/HL/NHLBI NIH HHS/United States
- R01 HL096647-01A2/HL/NHLBI NIH HHS/United States
- R01-HL094971/HL/NHLBI NIH HHS/United States
- R01 HL096647/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous