Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism - PubMed (original) (raw)
Modulation of dendritic spine development and plasticity by BDNF and vesicular trafficking: fundamental roles in neurodevelopmental disorders associated with mental retardation and autism
Christopher A Chapleau et al. J Neurodev Disord. 2009 Sep.
Abstract
The process of axonal and dendritic development establishes the synaptic circuitry of the central nervous system (CNS) and is the result of interactions between intrinsic molecular factors and the external environment. One growth factor that has a compelling function in neuronal development is the neurotrophin brain-derived neurotrophic factor (BDNF). BDNF participates in axonal and dendritic differentiation during embryonic stages of neuronal development, as well as in the formation and maturation of dendritic spines during postnatal development. Recent studies have also implicated vesicular trafficking of BDNF via secretory vesicles, and both secretory and endosomal trafficking of vesicles containing synaptic proteins, such as neurotransmitter and neurotrophin receptors, in the regulation of axonal and dendritic differentiation, and in dendritic spine morphogenesis. Several genes that are either mutated or deregulated in neurodevelopmental disorders associated with mental retardation have now been identified, and several mouse models of these disorders have been generated and characterized. Interestingly, abnormalities in dendritic and synaptic structure are consistently observed in human neurodevelopmental disorders associated with mental retardation, and in mouse models of these disorders as well. Abnormalities in dendritic and synaptic differentiation are thought to underlie altered synaptic function and network connectivity, thus contributing to the clinical outcome. Here, we review the roles of BDNF and vesicular trafficking in axonal and dendritic differentiation in the context of dendritic and axonal morphological impairments commonly observed in neurodevelopmental disorders associated with mental retardation.
Keywords: Autism; BDNF; Dendritic spine; Hippocampus; Mental retardation; Pyramidal neuron; Rett syndrome; Vesicle trafficking.
Figures
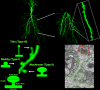
Fig. 1
The structure of dendritic spines of hippocampal pyramidal neurons. Using particle-mediated gene transfer (a.k.a. gene gun), organotypic slice cultures were transfected with cDNA coding for eYFP. Top panels: Laser-scanning confocal microscopy images of a pyramidal neuron in area CA1 are shown at different magnifications to illustrate the complexity of their dendritic arbor and the abundance of dendritic spines in secondary and tertiary branches. Bottom left panel: A maximum-intensity projection of _z-_stacks shows a dendritic segment studded with the most common spine morphologies, i.e. stubby, mushroom and thin. The cartoon illustrates the geometrical dimensions measured in individual spines to categorize them (adapted from Ref. 32). Bottom right panel: A mushroom dendritic spine (outlined in green) forms an asymmetric synapse with a single presynaptic terminal (outlined in red) in stratum radiatum of area CA1 in organotypic slice culture
Similar articles
- Transient receptor potential channels as novel effectors of brain-derived neurotrophic factor signaling: potential implications for Rett syndrome.
Amaral MD, Chapleau CA, Pozzo-Miller L. Amaral MD, et al. Pharmacol Ther. 2007 Feb;113(2):394-409. doi: 10.1016/j.pharmthera.2006.09.005. Epub 2006 Nov 21. Pharmacol Ther. 2007. PMID: 17118456 Free PMC article. Review. - Neurotrophin and Wnt signaling cooperatively regulate dendritic spine formation.
Hiester BG, Galati DF, Salinas PC, Jones KR. Hiester BG, et al. Mol Cell Neurosci. 2013 Sep;56:115-27. doi: 10.1016/j.mcn.2013.04.006. Epub 2013 Apr 30. Mol Cell Neurosci. 2013. PMID: 23639831 Free PMC article. - BDNF and TrkB in neuronal differentiation of Fmr1-knockout mouse.
Louhivuori V, Vicario A, Uutela M, Rantamäki T, Louhivuori LM, Castrén E, Tongiorgi E, Akerman KE, Castrén ML. Louhivuori V, et al. Neurobiol Dis. 2011 Feb;41(2):469-80. doi: 10.1016/j.nbd.2010.10.018. Epub 2010 Nov 1. Neurobiol Dis. 2011. PMID: 21047554 - Miniature synaptic transmission and BDNF modulate dendritic spine growth and form in rat CA1 neurones.
Tyler WJ, Pozzo-Miller L. Tyler WJ, et al. J Physiol. 2003 Dec 1;553(Pt 2):497-509. doi: 10.1113/jphysiol.2003.052639. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500767 Free PMC article. - Plasticity of dendritic spines: Molecular function and dysfunction in neurodevelopmental disorders.
Nishiyama J. Nishiyama J. Psychiatry Clin Neurosci. 2019 Sep;73(9):541-550. doi: 10.1111/pcn.12899. Epub 2019 Jul 8. Psychiatry Clin Neurosci. 2019. PMID: 31215705 Review.
Cited by
- Morphometric Analysis of Hippocampal and Neocortical Pyramidal Neurons in a Mouse Model of Late Onset Alzheimer's Disease.
Mehder RH, Bennett BM, Andrew RD. Mehder RH, et al. J Alzheimers Dis. 2020;74(4):1069-1083. doi: 10.3233/JAD-191067. J Alzheimers Dis. 2020. PMID: 32144984 Free PMC article. - Microtubule-Actin Crosslinking Factor 1 Is Required for Dendritic Arborization and Axon Outgrowth in the Developing Brain.
Ka M, Kim WY. Ka M, et al. Mol Neurobiol. 2016 Nov;53(9):6018-6032. doi: 10.1007/s12035-015-9508-4. Epub 2015 Nov 2. Mol Neurobiol. 2016. PMID: 26526844 Free PMC article. - Lighting a path: genetic studies pinpoint neurodevelopmental mechanisms in autism and related disorders.
Pescosolido MF, Yang U, Sabbagh M, Morrow EM. Pescosolido MF, et al. Dialogues Clin Neurosci. 2012 Sep;14(3):239-52. Dialogues Clin Neurosci. 2012. PMID: 23226950 Free PMC article. Review. - Dendritic spine dysgenesis in autism related disorders.
Phillips M, Pozzo-Miller L. Phillips M, et al. Neurosci Lett. 2015 Aug 5;601:30-40. doi: 10.1016/j.neulet.2015.01.011. Epub 2015 Jan 8. Neurosci Lett. 2015. PMID: 25578949 Free PMC article. Review. - Current Methodological Pitfalls and Caveats in the Assessment of Exercise-Induced Changes in Peripheral Brain-Derived Neurotrophic Factor: How Result Reproducibility Can Be Improved.
Nicolini C, Nelson AJ. Nicolini C, et al. Front Neuroergon. 2021 May 28;2:678541. doi: 10.3389/fnrgo.2021.678541. eCollection 2021. Front Neuroergon. 2021. PMID: 38235217 Free PMC article. Review.
References
Grants and funding
- P30 NS047466/NS/NINDS NIH HHS/United States
- P30 NS057098/NS/NINDS NIH HHS/United States
- P30 HD038985/HD/NICHD NIH HHS/United States
- R01 NS040593/NS/NINDS NIH HHS/United States
- R21 NS057780/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources