Does an interspinous device (Coflex) improve the outcome of decompressive surgery in lumbar spinal stenosis? One-year follow up of a prospective case control study of 60 patients - PubMed (original) (raw)
Clinical Trial
Does an interspinous device (Coflex) improve the outcome of decompressive surgery in lumbar spinal stenosis? One-year follow up of a prospective case control study of 60 patients
Alexander Richter et al. Eur Spine J. 2010 Feb.
Abstract
A number of interspinous process devices have recently been introduced to the lumbar spinal market as an alternative to conventional surgical procedures in the treatment of symptomatic lumbar stenosis. One of those "dynamic" devices is the Coflex device which has been already implanted worldwide more than 14,000 times. The aim of implanting this interspinous device is to unload the facet joints, restore foraminal height and provide stability in order to improve the clinical outcome of surgery. Published information is limited, and there are so far no data of comparison between the implant and traditional surgical approaches such as laminotomy. The purpose of our prospective study is to evaluate the surgical outcome of decompressive surgery in comparison to decompressive surgery and additional implantation of the Coflex interspinous Device. 60 patients who were all treated in the Spine Center of Klinikum Neustadt, Germany for a one or two level symptomatic LSS with decompressive surgery were included. Two groups were built. In Group one (UD) we treated 30 patients with decompression surgery alone and group two (CO) in 30 patients a Coflex device was additional implanted. Pre- and postoperatively disability and pain scores were measured using the Oswestry disability index (ODI), the Roland-Morris score (RMS), the visual analogue scale (VAS) and the pain-free walking distance (WD). Patients underwent postoperative assessments 3, 6 and 12 month including the above-mentioned scores as well as patient satisfaction. In both groups we could see a significant improve (p < 0.001) in the clinical outcome assessed in the ODI, in the RMS for evaluation of back pain, in the VAS and in the pain-free WD at all times of reinvestigation compared to base line. At 1-year follow up there were no statistically differences between both groups in all ascertained parameters including patient satisfaction and subjective operation decision. Because there is no current evidence of the efficacy of the Coflex device we need further data from randomized controlled studies for defining the indications for theses procedures. To the best of our knowledge this is the first prospective controlled study which compares surgical decompression of lumbar spinal stenosis with additional implanting of an interspinous Coflex device in the treatment of symptomatic LSS.
Figures
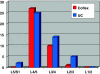
Fig. 1
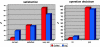
The distribution of the treated level in the Coflex-group and the undercutting-group (UC)
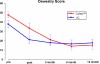
Fig. 2
Oswestry disability index preoperatively and at each follow up in the two treatment groups. All follow up scores are significant improved (p < 0.001) compared to base line with no significant differences between the treatments
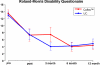
Fig. 3
Roland–Morris disability questionnaire preoperatively and at each follow up in the two treatment groups. All follow up scores are significant improved (p < 0.001) compared to base line with no significant differences between the treatments
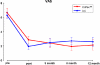
Fig. 4
Visual analogue scale preoperatively and at each follow up in the two treatment groups. All follow up scores show a significant reduction of pain (p < 0.001) compared to base line with no significant differences between the treatments
Fig. 5
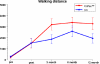
Walking distance over time. All patients had a significant prolonged walking distance (p < 0.001) with no significant main difference between the operation method was found
Fig. 6
Subjective operation decision and subjective satisfaction
Fig. 7
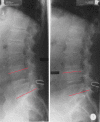
Postoperative flexion–extension radiographs after decompressive surgery and Coflex implantation in L4/5 showing the range of motion for the functional spinal unit
Comment in
- Lumbale Spinalkanalstenose - Verbessert ein interspinöser Spreizer die Ergebnisse der Dekompressionschirurgie?
Beck M. Beck M. Z Orthop Unfall. 2010 May;148(3):264. doi: 10.1055/s-0030-1255499. Epub 2010 Jun 21. Z Orthop Unfall. 2010. PMID: 20568048 German. No abstract available. - Evidence-based recommendations for spine surgery.
Fisher CG, Vaccaro AR, Whang PG, Prasad SK, Angevine PD, Mulpuri K, Thomas KC, Patel AA. Fisher CG, et al. Spine (Phila Pa 1976). 2011 Jun 15;36(14):E897-903. doi: 10.1097/BRS.0b013e31821c06d8. Spine (Phila Pa 1976). 2011. PMID: 21642806 No abstract available.
Similar articles
- Two-year follow-up after decompressive surgery with and without implantation of an interspinous device for lumbar spinal stenosis: a prospective controlled study.
Richter A, Halm HF, Hauck M, Quante M. Richter A, et al. J Spinal Disord Tech. 2014 Aug;27(6):336-41. doi: 10.1097/BSD.0b013e31825f7203. J Spinal Disord Tech. 2014. PMID: 22643187 Clinical Trial. - Prospective, randomized, multicenter study with 2-year follow-up to compare the performance of decompression with and without interlaminar stabilization.
Schmidt S, Franke J, Rauschmann M, Adelt D, Bonsanto MM, Sola S. Schmidt S, et al. J Neurosurg Spine. 2018 Apr;28(4):406-415. doi: 10.3171/2017.11.SPINE17643. Epub 2018 Jan 26. J Neurosurg Spine. 2018. PMID: 29372860 Clinical Trial. - Surgical treatment of the spinal stenosis with an interspinous distraction device: do we really restore the foraminal height?
Celik H, Derincek A, Koksal I. Celik H, et al. Turk Neurosurg. 2012;22(1):50-4. doi: 10.5137/1019-5149.JTN.4681-11.2. Turk Neurosurg. 2012. PMID: 22274971 - Is the interspinous process device safe and effective in elderly patients with lumbar degeneration? A systematic review and meta-analysis of randomized controlled trials.
Han B, Chen Y, Liang W, Yang Y, Ding Z, Yin P, Hai Y. Han B, et al. Eur Spine J. 2024 Mar;33(3):881-891. doi: 10.1007/s00586-023-08119-z. Epub 2024 Feb 12. Eur Spine J. 2024. PMID: 38342843 Review. - [The importance of interspinous spacers in the treatment of lumbar spinal stenosis].
Vinas-Rios JM, Arabmotlagh M, Rahim T, Schmidt S, Sellei RM, Rauschmann M. Vinas-Rios JM, et al. Orthopade. 2019 Oct;48(10):831-836. doi: 10.1007/s00132-019-03772-z. Orthopade. 2019. PMID: 31297556 Review. German.
Cited by
- Percutaneous interspinous spacer versus open decompression: a 2-year follow-up of clinical outcome and quality of life.
Beyer F, Yagdiran A, Neu P, Kaulhausen T, Eysel P, Sobottke R. Beyer F, et al. Eur Spine J. 2013 Sep;22(9):2015-21. doi: 10.1007/s00586-013-2790-9. Epub 2013 Apr 27. Eur Spine J. 2013. PMID: 23625306 Free PMC article. - Minimally invasive surgery for lumbar spinal stenosis.
Moojen WA, Van der Gaag NA. Moojen WA, et al. Eur J Orthop Surg Traumatol. 2016 Oct;26(7):681-4. doi: 10.1007/s00590-016-1828-1. Epub 2016 Sep 22. Eur J Orthop Surg Traumatol. 2016. PMID: 27659170 No abstract available. - IPD without bony decompression versus conventional surgical decompression for lumbar spinal stenosis: 2-year results of a double-blind randomized controlled trial.
Moojen WA, Arts MP, Jacobs WC, van Zwet EW, van den Akker-van Marle ME, Koes BW, Vleggeert-Lankamp CL, Peul WC; Leiden The Hague Spine Intervention Prognostic Study Group (SIPS). Moojen WA, et al. Eur Spine J. 2015 Oct;24(10):2295-305. doi: 10.1007/s00586-014-3748-2. Epub 2015 Jan 14. Eur Spine J. 2015. PMID: 25586759 Clinical Trial. - Role of lumbar interspinous distraction on the neural elements.
Alfieri A, Gazzeri R, Prell J, Scheller C, Rachinger J, Strauss C, Schwarz A. Alfieri A, et al. Neurosurg Rev. 2012 Oct;35(4):477-84; discussion 484. doi: 10.1007/s10143-012-0394-1. Epub 2012 May 2. Neurosurg Rev. 2012. PMID: 22549123 Review. - Spinal Stenosis in the Absence of Spondylolisthesis: Can Interlaminar Stabilization at Single and Multi-levels Provide Sustainable Relief?
Abjornson C, Yoon BV, Callanan T, Shein D, Grinberg S, Cammisa FP. Abjornson C, et al. Int J Spine Surg. 2018 Mar 30;12(1):64-69. doi: 10.14444/5011. eCollection 2018 Jan. Int J Spine Surg. 2018. PMID: 30280085 Free PMC article.
References
- Bertagnoli R (2007) Coflex interspinous implant : motion preserving treatment in lumbar degenerative stenosis patients-min. 1-Y. Results. Berlin, SAS. SAS Global Symposium on Motion Preservation Technology 2007 Ref Type: report
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical