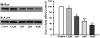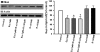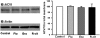Chronic treatment with escitalopram but not R-citalopram translocates Galpha(s) from lipid raft domains and potentiates adenylyl cyclase: a 5-hydroxytryptamine transporter-independent action of this antidepressant compound - PubMed (original) (raw)
Comparative Study
. 2010 Mar;332(3):977-84.
doi: 10.1124/jpet.109.162644. Epub 2009 Dec 8.
Affiliations
- PMID: 19996298
- PMCID: PMC2835448
- DOI: 10.1124/jpet.109.162644
Comparative Study
Chronic treatment with escitalopram but not R-citalopram translocates Galpha(s) from lipid raft domains and potentiates adenylyl cyclase: a 5-hydroxytryptamine transporter-independent action of this antidepressant compound
Lanqiu Zhang et al. J Pharmacol Exp Ther. 2010 Mar.
Abstract
Chronic antidepressant treatment has been shown to increase adenylyl cyclase activity, in part, due to translocation of Galpha(s) from lipid rafts to a nonraft fraction of the plasma membrane where they engage in a more facile stimulation of adenylyl cyclase. This effect holds for multiple classes of antidepressants, and for serotonin uptake inhibitors, it occurs in the absence of the serotonin transporter. In the present study, we examined the change in the amount of Galpha(s) in lipid raft and whole cell lysate after exposing C6 cells to escitalopram. The results showed that chronic (but not acute) escitalopram decreased the content of Galpha(s) in lipid rafts, whereas there was no change in overall Galpha(s) content. These effects were drug dose- and exposure time-dependent. Although R-citalopram has been reported to antagonize some effects of escitalopram, this compound was without effect on Galpha(s) localization in lipid rafts, and R-citalopram did not inhibit these actions of escitalopram. Escitalopram treatment increased cAMP accumulation, and this seemed due to increased coupling between Galpha(s) and adenylyl cyclase. Thus, escitalopram is potent, rapid and efficacious in translocating Galpha(s) from lipid rafts, and this effect seems to occur independently of 5-hydroxytryptamine transporters. Our results suggest that, although antidepressants display distinct affinities for well identified targets (e.g., monoamine transporters), several presynaptic and postsynaptic molecules are probably modified during chronic antidepressant treatment, and these additional targets may be required for clinical efficacy of these drugs.
Figures
Fig. 1.
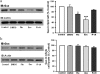
Effect of escitalopram and _R_-citalopram on the cellular and lipid rafts content of Gαs. a, C6 cells were treated chronically with fluoxetine (Flu), escitalopram (Esc), and _R_-citalopram (_R_-cit) (at 10 μM for 3 days). The detergent-insoluble lipid rafts were obtained by sucrose density gradients fractionation and analyzed by SDS-polyacrylamide gel electrophoresis and immunoblot for Gαs content. A representative blot of Gαs protein is shown (top); the same blot was reprobed for actin (bottom). The figure shows the percentage of change in Gαs protein above control in the lipid raft membrane fractions from three independent experiments. b, Gαs content in whole cell lysates detected with immunoblots (top) in comparison with actin (bottom). The figure is a quantification of Gαs protein in whole cell lysates and is presented as a percentage of control (n = 3). Data were analyzed by one-way ANOVA followed by Fisher's LSD test for post hoc comparisons of means. Data are represented as mean ± S.E.M. (∗, p < 0.05; ∗∗∗, p < 0.001 versus control).
Fig. 2.
Escitalopram-induced translocation of Gαs from lipid rafts is dose-dependent. C6 cells were treated with 0.2, 1, 5, and 10 μM escitalopram, respectively, for 3 days, and detergent-insoluble lipid rafts were obtained as described in Fig. 1. The quantity of Gαs in lipid rafts was determined by Western blotting. A representative blot of Gαs protein is shown (top) as well as actin protein (bottom). The figure shows the percentage of change in Gαs protein above control in the lipid raft membrane fractions (n = 4). Data were analyzed by one-way ANOVA followed by Fisher's LSD test for post hoc comparisons of means. Data are represented as mean ± S.E.M. (∗∗, p < 0.01; ∗∗∗, p < 0.001 versus control).
Fig. 3.
Time course for escitalopram-induced translocation of Gαs from lipid rafts. C6 cells were cultured for 5 days and drug was initiated in the final 1, 3, or 5 days of this period with escitalopram at the indicated dose. Cells treated for 1 day were grown for 4 days and treated for the final 24 h, and those treated for 3 days were grown for 2 days before escitalopram treatment. Subsequent to treatment, lipid raft fractions were isolated as described above. Data were analyzed by one-way ANOVA followed by Fisher's LSD test for post hoc comparisons of means. Data are represented as mean ± S.E.M. (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001 versus control). a to c, Gαs protein contents in lipid rafts were detected by immunoblot (top) and compared with actin as a loading control (bottom) after C6 cells were treated with 1 μM escitalopram (Esc) for 1, 3, and 5 days, respectively (1 and 2 μM Esc; n = 4; 5 μM Esc, n = 3). C, control. d, compiled time course data for multiple experiments.
Fig. 4.
_R_-Citalopram does not inhibit escitalopram-induced translocation of Gαs from lipid rafts. C6 cells were treated with escitalopram (1 μM) alone or together with _R_-citalopram (1 or 5 μM) for 3 days. After treatment, lipid raft fractions were isolated as described above. A representative immunoblot shows Gαs protein (top) and actin protein (bottom) in lipid rafts. The figure shows the percentage of change in Gαs protein above control in the lipid raft membrane fractions (n = 5). Data were analyzed by one-way ANOVA followed by Fisher's LSD test for post hoc comparisons of means. Data are represented as mean ± S.E.M. (∗, p < 0.05 versus control).
Fig. 5.
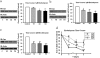
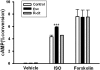
Chronic escitalopram (Esc) treatment potentiates G protein-coupled receptor-activated cAMP production. C6 cells grown in 12-well plates were exposed to 10 μM escitalopram or _R_-citalopram after plating for a period of 3 days (n = 3). On day 2, cells were also incubated for 24 h with 4 μCi/ml [2,8-3H]adenine to label the total pool of cellular ATP. Cells were washed with serum-free Dulbecco's modified Eagle's medium (without Esc or [3H]adenine) and incubated with or without 10 μM isoproterenol or 100 μM forskolin at 37°C for 30 min. cAMP production was expressed as [3H]cAMP relative to [3H]adenine incorporated into cells (percentage of conversion of [3H]adenine to [3H]cAMP). Data presented are the mean values ± S.E.M. from three independent experiments performed in triplicate. Data were analyzed by one-way ANOVA followed by Fisher's LSD test for post hoc comparisons of means (∗∗∗, p = 0.001 versus control).
Fig. 6.
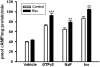
Escitalopram treatment increases on GTPγS, fluoride and isoproterenol-induced adenylyl cyclase activity in C6 membranes. C6 cell membranes were made from cells exposed to medium containing 10 μM escitalopram for 3 days (n = 4). The membranes were assayed for adenylyl cyclase activity as described under Materials and Methods. Data represented as mean ± S.E.M. of triplicate determinations from one of four similar experiments are shown. Data were analyzed by one-way ANOVA followed by Fisher's LSD test for post hoc comparisons of means (∗∗, p < 0.01; ∗∗∗, P < 0.001 versus control).
Fig. 7.
C6 cells were treated chronically with fluoxetine (Flu), escitalopram (Esc), and _R_-citalopram (_R_-cit) (at 10 μM for 3 days). Total cellular membranes were obtained as described under Materials and Methods. The quantity of ACVI in the membrane was determined by Western blotting. A representative immunoblot shows ACVI (top) and actin protein (bottom) in membrane. The figure is a quantification of ACVI protein, represented as a percentage of control (n = 3). Data were analyzed by one-way ANOVA followed by Fisher's LSD test for post hoc comparisons of means. Data are represented as the mean ± S.E.M.
Similar articles
- Differential effects of antidepressants escitalopram versus lithium on Gs alpha membrane relocalization.
Donati RJ, Schappi J, Czysz AH, Jackson A, Rasenick MM. Donati RJ, et al. BMC Neurosci. 2015 Jul 11;16:40. doi: 10.1186/s12868-015-0178-y. BMC Neurosci. 2015. PMID: 26162823 Free PMC article. - Antidepressants Accumulate in Lipid Rafts Independent of Monoamine Transporters to Modulate Redistribution of the G Protein, Gαs.
Erb SJ, Schappi JM, Rasenick MM. Erb SJ, et al. J Biol Chem. 2016 Sep 16;291(38):19725-19733. doi: 10.1074/jbc.M116.727263. Epub 2016 Jul 18. J Biol Chem. 2016. PMID: 27432886 Free PMC article. - Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling.
Allen JA, Yu JZ, Dave RH, Bhatnagar A, Roth BL, Rasenick MM. Allen JA, et al. Mol Pharmacol. 2009 Nov;76(5):1082-93. doi: 10.1124/mol.109.060160. Epub 2009 Aug 20. Mol Pharmacol. 2009. PMID: 19696145 Free PMC article. - [Escitalopram: a selective inhibitor and allosteric modulator of the serotonin transporter].
Mnie-Filali O, El Mansari M, Scarna H, Zimmer L, Sánchez C, Haddjeri N. Mnie-Filali O, et al. Encephale. 2007 Dec;33(6):965-72. doi: 10.1016/j.encep.2007.11.001. Epub 2007 Dec 11. Encephale. 2007. PMID: 18789789 Review. French. - The pharmacology of citalopram enantiomers: the antagonism by R-citalopram on the effect of S-citalopram.
Sánchez C. Sánchez C. Basic Clin Pharmacol Toxicol. 2006 Aug;99(2):91-5. doi: 10.1111/j.1742-7843.2006.pto_295.x. Basic Clin Pharmacol Toxicol. 2006. PMID: 16918708 Review.
Cited by
- Accumulation of an antidepressant in vesiculogenic membranes of yeast cells triggers autophagy.
Chen J, Korostyshevsky D, Lee S, Perlstein EO. Chen J, et al. PLoS One. 2012;7(4):e34024. doi: 10.1371/journal.pone.0034024. Epub 2012 Apr 18. PLoS One. 2012. PMID: 22529904 Free PMC article. - Differential effects of antidepressants escitalopram versus lithium on Gs alpha membrane relocalization.
Donati RJ, Schappi J, Czysz AH, Jackson A, Rasenick MM. Donati RJ, et al. BMC Neurosci. 2015 Jul 11;16:40. doi: 10.1186/s12868-015-0178-y. BMC Neurosci. 2015. PMID: 26162823 Free PMC article. - Disruption of lipid-raft localized Gαs/tubulin complexes by antidepressants: a unique feature of HDAC6 inhibitors, SSRI and tricyclic compounds.
Singh H, Wray N, Schappi JM, Rasenick MM. Singh H, et al. Neuropsychopharmacology. 2018 Jun;43(7):1481-1491. doi: 10.1038/s41386-018-0016-x. Epub 2018 Feb 5. Neuropsychopharmacology. 2018. PMID: 29463911 Free PMC article. Retracted. - Fish oil and depression: The skinny on fats.
Burhani MD, Rasenick MM. Burhani MD, et al. J Integr Neurosci. 2017;16(s1):S115-S124. doi: 10.3233/JIN-170072. J Integr Neurosci. 2017. PMID: 29254106 Free PMC article. Review. - Regulation of monoamine transporters and receptors by lipid microdomains: implications for depression.
Liu JJ, Hezghia A, Shaikh SR, Cenido JF, Stark RE, Mann JJ, Sublette ME. Liu JJ, et al. Neuropsychopharmacology. 2018 Oct;43(11):2165-2179. doi: 10.1038/s41386-018-0133-6. Epub 2018 Jun 28. Neuropsychopharmacology. 2018. PMID: 30022062 Free PMC article. Review.
References
- Allen JA, Yu JZ, Donati RJ, Rasenick MM. (2005) Beta-adrenergic receptor stimulation promotes G alpha s internalization through lipid rafts: a study in living cells. Mol Pharmacol 67:1493–1504 - PubMed
- Bianchi M, Shah AJ, Fone KC, Atkins AR, Dawson LA, Heidbreder CA, Hows ME, Hagan JJ, Marsden CA. (2009) Fluoxetine administration modulates the cytoskeletal microtubular system in the rat hippocampus. Synapse 63:359–364 - PubMed
- Chen F, Larsen MB, Neubauer HA, Sánchez C, Plenge P, Wiborg O. (2005) Characterization of an allosteric citalopram-binding site at the serotonin transporter. J Neurochem 92:21–28 - PubMed
- Chen J, Rasenick MM. (1995a) Chronic antidepressant treatment facilitates G protein activation of adenylyl cyclase without altering G protein content. J Pharmacol Exp Ther 275:509–517 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases