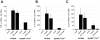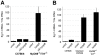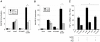Innate immune recognition of Yersinia pseudotuberculosis type III secretion - PubMed (original) (raw)
Innate immune recognition of Yersinia pseudotuberculosis type III secretion
Victoria Auerbuch et al. PLoS Pathog. 2009 Dec.
Abstract
Specialized protein translocation systems are used by many bacterial pathogens to deliver effector proteins into host cells that interfere with normal cellular functions. How the host immune system recognizes and responds to this intrusive event is not understood. To address these questions, we determined the mammalian cellular response to the virulence-associated type III secretion system (T3SS) of the human pathogen Yersinia pseudotuberculosis. We found that macrophages devoid of Toll-like receptor (TLR) signaling regulate expression of 266 genes following recognition of the Y. pseudotuberculosis T3SS. This analysis revealed two temporally distinct responses that could be separated into activation of NFkappaB- and type I IFN-regulated genes. Extracellular bacteria were capable of triggering these signaling events, as inhibition of bacterial uptake had no effect on the ensuing innate immune response. The cytosolic peptidoglycan sensors Nod1 and Nod2 and the inflammasome component caspase-1 were not involved in NFkappaB activation following recognition of the Y. pseudotuberculosis T3SS. However, caspase-1 was required for secretion of the inflammatory cytokine IL-1beta in response to T3SS-positive Y. pseudotuberculosis. In order to characterize the bacterial requirements for induction of this novel TLR-, Nod1/2-, and caspase-1-independent response, we used Y. pseudotuberculosis strains lacking specific components of the T3SS. Formation of a functional T3SS pore was required, as bacteria expressing a secretion needle, but lacking the pore-forming proteins YopB or YopD, did not trigger these signaling events. However, nonspecific membrane disruption could not recapitulate the NFkappaB signaling triggered by Y. pseudotuberculosis expressing a functional T3SS pore. Although host cell recognition of the T3SS did not require known translocated substrates, the ensuing response could be modulated by effectors such as YopJ and YopT, as YopT amplified the response, while YopJ dampened it. Collectively, these data suggest that combined recognition of the T3SS pore and YopBD-mediated delivery of immune activating ligands into the host cytosol informs the host cell of pathogenic challenge. This leads to a unique, multifactorial response distinct from the canonical immune response to a bacterium lacking a T3SS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
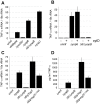
Figure 1. Extracellular Y. pseudotuberculosis expressing a functional T3SS translocator induces TLR-independent TNF-α production.
MyD88−/−/Trif−/− macrophages were infected with Y. pseudotuberculosis and tnfa mRNA levels (normalized to 18s rRNA) (A–C) and TNF-α protein levels (D) were quantified two hours post-inoculation. (B) Phagocytosis was inhibited by pre-incubating macrophages with cytochalasin D (cytD). The average of two to nine independent experiments ± standard error of the mean (sem) is shown.
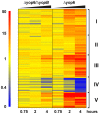
Figure 2. Y. pseudotuberculosis expressing a functional T3SS translocator induces robust macrophage gene expression.
MyD88−/−/Trif−/− macrophages were infected with Y. pseudotuberculosis Δyop6, Δ6/Δ_yopB_, or were left uninfected. Total RNA was isolated and Affymetrix GeneChip Mouse Genome 430 2.0 arrays were used to probe the relative gene expression profiles for each experimental condition. The expression of 266 murine genes (represented by 340 probe sets) was regulated by Y. pseudotuberculosis Δyop6 at least four-fold (p<0.05) more than by Y. pseudotuberculosis Δ6/Δ_yopB_ during at least one time point. The probe sets were clustered into a heat map according to the Pearson correlation. From left to right, the 45 minute, two hour, and four hour time points are shown for each bacterial strain. The colorbar ranges from zero to 50-fold change in gene expression compared to the uninfected condition where red represents upregulated genes, yellow represents no change from uninfected, and blue represents downregulated genes.
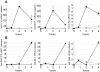
Figure 3. NFκB and type I IFN regulated genes exhibit different kinetic profiles following infection with T3SS translocator-positive Y. pseudotuberculosis.
(A) Three NFκB-regulated genes and (B) three type I IFN-induced genes were selected from among the 266 genes identified by the microarray analysis described in Fig. 2. Shown is the average fold induction of the given probe set over the uninfected condition from Y. pseudotuberculosis Δyop6-infected (diamonds) and Δ6/Δ_yopB_-infected (circles) BMDMs ± sem.
Figure 4. T3SS translocator-positive and –negative Y. pseudotuberculosis can be differentiated by a TLR-independent recognition pathway.
C57Bl/6 and MyD88−/−/Trif−/− macrophages were infected with Y. pseudotuberculosis and total RNA isolated at 2 hours post-inoculation. (A) tnfa, (B) il10, and (C) axud1 mRNA levels were quantified (normalized to 18s rRNA). Data represents average±sem from three independent experiments.
Figure 5. Tolerization of TLR signaling in wildtype macrophages relieves egr1 repression during infection with Y. pseudotuberculosis.
C57Bl/6 and MyD88−/−/Trif−/− macrophages were (A) infected with Y. pseudotuberculosis and total RNA isolated at 2 hours post-inoculation or (B) were first incubated with heat-killed Y. pseudotuberculosis overnight (tolerized) or were left untreated. The tolerized or untreated macrophages were then infected with live Y. pseudotuberculosis Δyop6 and total RNA isolated two hours post-inoculation. Egr1 mRNA levels (normalized to 18s rRNA) were quantified. Data represents average ± sem from two independent experiments.
Figure 6. Y. pseudotuberculosis triggers NFκB activation independently of Nod1 and Nod2.
(A–B) 293T cells were transfected with plasmids encoding siRNA against Nod1 or EGFP (A) or Rip2 or LacZ (B) as well as a plasmid expressing an NFκB luciferase reporter gene. The transfected cells were then infected with Y. pseudotuberculosis or treated with a synthetic Nod1 ligand, C12-iE-DAP, that is able to access the cell cytosol. Four hours post-inoculation or post-treatment, NFκB activation was quantified by measuring luminescence. Data shown is the average fold NFκB activation (over uninfected/untreated) ± sem from one independent, representative experiment and each experiment was repeated for a total of three (Nod1) to four (Rip2) replicates. * Statistically significant decrease in C12-iE-DAP-induced NFκB activation according to the student t-Test. P<0.01 for Nod1 shRNA compared to EGFP control. P<0.03 for Rip2 shRNA compared to LacZ control. There was no statistical difference in NFκB activation induced by Y. pseudotuberculosis_ Δyop6 with either Nod1 (p>0.2) or Rip2 (p>0.3) knockdown. (C) MyD88−/−, MyD88−/−/Rip2−/−, and MyD88−/−/Trif−/− macrophages were infected with Y. pseudotuberculosis Δyop6 or Δ6/Δ_yopB and tnfa mRNA levels (normalized to 18s rRNA) were measured two hours post-inoculation. Data shown is the average ± sem from one independent, representative experiment, which was repeated for a total of two replicates. * The only statistically significant decrease in tnfa mRNA levels compared to the Trif+/+/Rip2+/+ control cells was observed for the Y. pseudotuberculosis Δ6/Δ_yopB_ strain in Trif−/−/Rip2+/+ cells (student t-Test, p<0.002).
Figure 7. Neither introduction of type III secreted molecules into the cytosol of 293T cells nor nonspecific membrane disruption induce IL-8 production.
(A) 293T cells were infected with different MOIs of live Y. pseudotuberculosis for two hours. (B) Filtrate from Y. pseudotuberculosis Δyop6 or Δ_yscNU_ culture grown under type III secretion-inducing conditions was scrape-loaded into 293T cells for 5–10 minutes and the scrape-loaded cells incubated for an additional two hours. The Δ_yscNU_ strain does not express a T3SS, does not secrete any T3SS cargo into the culture supernatant, and is the negative control for scrape-loading Δyop6 culture filtrate. Alternatively, varying amounts of heat-killed and lysed Y. pseudotuberculosis or buffer were scrape-loaded into 293T cells for 5–10 minutes and the scrape-loaded cells incubated for an additional two hours. Total RNA was isolated and il8 mRNA levels (normalized to 18s rRNA) were quantified. Data shown is the average±sem from one independent, representative experiment and each experiment was repeated for a total of two (A) to three (B) replicates. * Statistically significant increase (p<0.0001) in il8 levels according to the student t-Test compared to uninfected (A) or scrape only (B) controls.
Figure 8. Activation of cytosolic innate immune signaling by the Y. pseudotuberculosis T3SS requires a YopBD-mediated translocation event.
Y. pseudotuberculosis expressing a functional T3SS translocator triggers NFκB and type I IFN innate immune signaling independently of T3SS effector proteins. This response requires the pore-forming proteins YopBD and cannot be recapitulated by nonspecific membrane disruption nor by introducing Y. pseudotuberculosis T3SS secreted molecules into host cells independently of the YopBD pore. We propose that both (1) YopBD-mediated membrane insertion or pore formation
and
(2) cytosolic entry of multiple immune activating molecules capable of triggering different transcriptional events are required for full activation of the innate immune response to the Y. pseudotuberculosis T3SS.
Similar articles
- Yersinia pseudotuberculosis YopJ Limits Macrophage Response by Downregulating COX-2-Mediated Biosynthesis of PGE2 in a MAPK/ERK-Dependent Manner.
Sheppe AEF, Santelices J, Czyz DM, Edelmann MJ. Sheppe AEF, et al. Microbiol Spectr. 2021 Sep 3;9(1):e0049621. doi: 10.1128/Spectrum.00496-21. Epub 2021 Jul 28. Microbiol Spectr. 2021. PMID: 34319170 Free PMC article. - Impact of host membrane pore formation by the Yersinia pseudotuberculosis type III secretion system on the macrophage innate immune response.
Kwuan L, Adams W, Auerbuch V. Kwuan L, et al. Infect Immun. 2013 Mar;81(3):905-14. doi: 10.1128/IAI.01014-12. Epub 2013 Jan 7. Infect Immun. 2013. PMID: 23297383 Free PMC article. - Inflammasome activation in response to the Yersinia type III secretion system requires hyperinjection of translocon proteins YopB and YopD.
Zwack EE, Snyder AG, Wynosky-Dolfi MA, Ruthel G, Philip NH, Marketon MM, Francis MS, Bliska JB, Brodsky IE. Zwack EE, et al. mBio. 2015 Feb 17;6(1):e02095-14. doi: 10.1128/mBio.02095-14. mBio. 2015. PMID: 25691590 Free PMC article. - Yersinia type III effectors perturb host innate immune responses.
Pha K, Navarro L. Pha K, et al. World J Biol Chem. 2016 Feb 26;7(1):1-13. doi: 10.4331/wjbc.v7.i1.1. World J Biol Chem. 2016. PMID: 26981193 Free PMC article. Review. - Role of the Yersinia pseudotuberculosis Virulence Plasmid in Pathogen-Phagocyte Interactions in Mesenteric Lymph Nodes.
Bliska JB, Brodsky IE, Mecsas J. Bliska JB, et al. EcoSal Plus. 2021 Dec 15;9(2):eESP00142021. doi: 10.1128/ecosalplus.ESP-0014-2021. Epub 2021 Oct 27. EcoSal Plus. 2021. PMID: 34910573 Free PMC article. Review.
Cited by
- Caspase-11 activation in response to bacterial secretion systems that access the host cytosol.
Casson CN, Copenhaver AM, Zwack EE, Nguyen HT, Strowig T, Javdan B, Bradley WP, Fung TC, Flavell RA, Brodsky IE, Shin S. Casson CN, et al. PLoS Pathog. 2013;9(6):e1003400. doi: 10.1371/journal.ppat.1003400. Epub 2013 Jun 6. PLoS Pathog. 2013. PMID: 23762026 Free PMC article. - C. elegans detects pathogen-induced translational inhibition to activate immune signaling.
Dunbar TL, Yan Z, Balla KM, Smelkinson MG, Troemel ER. Dunbar TL, et al. Cell Host Microbe. 2012 Apr 19;11(4):375-86. doi: 10.1016/j.chom.2012.02.008. Cell Host Microbe. 2012. PMID: 22520465 Free PMC article. - Induction and function of IFNβ during viral and bacterial infection.
Nagarajan U. Nagarajan U. Crit Rev Immunol. 2011;31(6):459-74. doi: 10.1615/critrevimmunol.v31.i6.20. Crit Rev Immunol. 2011. PMID: 22321107 Free PMC article. Review. - Iron availability and oxygen tension regulate the Yersinia Ysc type III secretion system to enable disseminated infection.
Hooker-Romero D, Mettert E, Schwiesow L, Balderas D, Alvarez PA, Kicin A, Gonzalez AL, Plano GV, Kiley PJ, Auerbuch V. Hooker-Romero D, et al. PLoS Pathog. 2019 Dec 23;15(12):e1008001. doi: 10.1371/journal.ppat.1008001. eCollection 2019 Dec. PLoS Pathog. 2019. PMID: 31869388 Free PMC article. - Pathogen-derived effectors trigger protective immunity via activation of the Rac2 enzyme and the IMD or Rip kinase signaling pathway.
Boyer L, Magoc L, Dejardin S, Cappillino M, Paquette N, Hinault C, Charriere GM, Ip WK, Fracchia S, Hennessy E, Erturk-Hasdemir D, Reichhart JM, Silverman N, Lacy-Hulbert A, Stuart LM. Boyer L, et al. Immunity. 2011 Oct 28;35(4):536-49. doi: 10.1016/j.immuni.2011.08.015. Epub 2011 Oct 20. Immunity. 2011. PMID: 22018470 Free PMC article.
References
- Magalhaes JG, Tattoli I, Girardin SE. The intestinal epithelial barrier: how to distinguish between the microbial flora and pathogens. Semin Immunol. 2007;19:106–115. - PubMed
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
- Rakoff-Nahoum S, Hao L, Medzhitov R. Role of toll-like receptors in spontaneous commensal-dependent colitis. Immunity. 2006;25:319–329. - PubMed
- Gewirtz AT, Navas TA, Lyons S, Godowski PJ, Madara JL. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J Immunol. 2001;167:1882–1885. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources