A truncating mutation of TRAPPC9 is associated with autosomal-recessive intellectual disability and postnatal microcephaly - PubMed (original) (raw)
A truncating mutation of TRAPPC9 is associated with autosomal-recessive intellectual disability and postnatal microcephaly
Ganeshwaran H Mochida et al. Am J Hum Genet. 2009 Dec.
Abstract
Although autosomal genes are increasingly recognized as important causes of intellectual disability, very few of them are known. We identified a genetic locus for autosomal-recessive nonsyndromic intellectual disability associated with variable postnatal microcephaly through homozygosity mapping of a consanguineous Israeli Arab family. Sequence analysis of genes in the candidate interval identified a nonsense nucleotide change in the gene that encodes TRAPPC9 (trafficking protein particle complex 9, also known as NIBP), which has been implicated in NF-kappaB activation and possibly in intracellular protein trafficking. TRAPPC9 is highly expressed in the postmitotic neurons of the cerebral cortex, and MRI analysis of affected patients shows defects in axonal connectivity. This suggests essential roles of TRAPPC9 in human brain development, possibly through its effect on NF-kappaB activation and protein trafficking in the postmitotic neurons of the cerebral cortex.
Figures
Figure 1
Brain MRI of a Patient with a TRAPPC9 Mutation (A) T1-weighted axial image of Patient 2 (MC-6102) reveals microcephaly but a normal gyral pattern of the cerebral cortex. The cerebral white matter is reduced. Scale bar is 2 cm for all images. (B) T1-weighted sagittal image of the same patient is remarkable for a thin but fully formed corpus callosum and mild hypoplasia of the inferior cerebellar vermis. (C) T1-weighted axial image of an age-matched control individual. (D) T1-weighted sagittal image of an age-matched control individual.
Figure 2
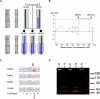
Mapping and Cloning of the Causative Gene (A) Pedigree and haplotype of the distal chromosome 8 of the family studied. There is an area of shared homozygosity in all three affected children (box). This identified the candidate region as defined by markers D8S272 and D8S1744. (B) A graph showing multipoint LOD scores of the same region of chromosome 8. LOD scores above 3.0 were achieved between D8S272 and D8S1744. (C) Sequencing of candidate genes within the interval revealed a nonsense nucleotide change in the exon 7 of the TRAPPC9 gene. The C to T change (arrow) in patients creates a stop codon (red shaded box) and is expected to result in R to X change on the amino acid level. (D) Western blot analysis of the lymphoblasts from the patients and control individuals. A single band was detected by Trappc9 antibody (green) in protein extracts from three different control lymphoblasts (C1, C2, and C3). This band was not detected in lymphoblast extracts from Patient 1 or Patient 2 (P1 and P2, respectively), despite the fact that a higher amount of protein was loaded compared to the control samples. Red represents a loading control (β-actin).
Figure 3
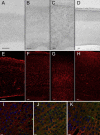
In Situ Hybridization and Immunofluorescence Studies of TRAPPC9 in the Developing Mouse and Human Brain (A) In E14 mouse brain, a low level of Trappc9 RNA expression was seen throughout the cerebral mantle (scale bar in [A]–[H] = 100 μm). (B) In P0 mouse brain, there was a slightly higher level of RNA expression in the cerebral mantle, with the exception of the developing white matter, which is devoid of staining. (C) In adult mouse cerebral cortex, there is a moderate to high level of RNA expression in cortical neurons of all layers. (D) In human brain at 11.5 weeks, a moderate level of TRAPPC9 RNA expression is seen in the cortical plate. (E) In E15 mouse brain, Trappc9 protein could be detected throughout the cerebral cortex, with increased accumulation in the cortical plate. (F) In P3 mouse brain, Trappc9 protein was detected in the cell bodies of cortical plate neurons and in cells within the subventricular zone. (G) Neuronal accumulation of Trappc9 continued in the P8 cerebral cortex, with higher levels of signal in the large pyramidal neurons of layer V. (H) Trappc9 immunofluorescence persisted in adult mouse cortical neurons. (I) Confocal microscopy of mouse cortical plate revealed distinct subcellular locations for Trappc9 (red) and the Golgi apparatus marker GM130 (green) (scale bar in [I]–[K] = 10 μm). (J) Trappc9 (red) did not colocalize with Rab7 (green), a marker of late endosomes and lysosomes. (K) Trappc9 (red) did not colocalize with transferrin receptor (green), a marker for early endosomes.
Similar articles
- A homozygous splice site mutation in TRAPPC9 causes intellectual disability and microcephaly.
Kakar N, Goebel I, Daud S, Nürnberg G, Agha N, Ahmad A, Nürnberg P, Kubisch C, Ahmad J, Borck G. Kakar N, et al. Eur J Med Genet. 2012 Dec;55(12):727-31. doi: 10.1016/j.ejmg.2012.08.010. Epub 2012 Aug 30. Eur J Med Genet. 2012. PMID: 22989526 - Identification of a novel homozygous TRAPPC9 gene mutation causing non-syndromic intellectual disability, speech disorder, and secondary microcephaly.
Abbasi AA, Blaesius K, Hu H, Latif Z, Picker-Minh S, Khan MN, Farooq S, Khan MA, Kaindl AM. Abbasi AA, et al. Am J Med Genet B Neuropsychiatr Genet. 2017 Dec;174(8):839-845. doi: 10.1002/ajmg.b.32602. Epub 2017 Oct 14. Am J Med Genet B Neuropsychiatr Genet. 2017. PMID: 29031008 - Identification of mutations in TRAPPC9, which encodes the NIK- and IKK-beta-binding protein, in nonsyndromic autosomal-recessive mental retardation.
Mir A, Kaufman L, Noor A, Motazacker MM, Jamil T, Azam M, Kahrizi K, Rafiq MA, Weksberg R, Nasr T, Naeem F, Tzschach A, Kuss AW, Ishak GE, Doherty D, Ropers HH, Barkovich AJ, Najmabadi H, Ayub M, Vincent JB. Mir A, et al. Am J Hum Genet. 2009 Dec;85(6):909-15. doi: 10.1016/j.ajhg.2009.11.009. Am J Hum Genet. 2009. PMID: 20004765 Free PMC article. - Emerging role of NIK/IKK2-binding protein (NIBP)/trafficking protein particle complex 9 (TRAPPC9) in nervous system diseases.
Bodnar B, DeGruttola A, Zhu Y, Lin Y, Zhang Y, Mo X, Hu W. Bodnar B, et al. Transl Res. 2020 Oct;224:55-70. doi: 10.1016/j.trsl.2020.05.001. Epub 2020 May 17. Transl Res. 2020. PMID: 32434006 Free PMC article. Review. - A recessive syndrome of intellectual disability, moderate overgrowth, and renal dysplasia predisposing to Wilms tumor is caused by a mutation in FIBP gene.
Akawi N, Ben-Salem S, Lahti L, Partanen J, Ali BR, Al-Gazali L. Akawi N, et al. Am J Med Genet A. 2016 Aug;170(8):2111-8. doi: 10.1002/ajmg.a.37741. Epub 2016 May 17. Am J Med Genet A. 2016. PMID: 27183861 Review.
Cited by
- Autosomal recessive mental retardation: homozygosity mapping identifies 27 single linkage intervals, at least 14 novel loci and several mutation hotspots.
Kuss AW, Garshasbi M, Kahrizi K, Tzschach A, Behjati F, Darvish H, Abbasi-Moheb L, Puettmann L, Zecha A, Weissmann R, Hu H, Mohseni M, Abedini SS, Rajab A, Hertzberg C, Wieczorek D, Ullmann R, Ghasemi-Firouzabadi S, Banihashemi S, Arzhangi S, Hadavi V, Bahrami-Monajemi G, Kasiri M, Falah M, Nikuei P, Dehghan A, Sobhani M, Jamali P, Ropers HH, Najmabadi H. Kuss AW, et al. Hum Genet. 2011 Feb;129(2):141-8. doi: 10.1007/s00439-010-0907-3. Epub 2010 Nov 10. Hum Genet. 2011. PMID: 21063731 - A novel deletion mutation in the TUSC3 gene in a consanguineous Pakistani family with autosomal recessive nonsyndromic intellectual disability.
Khan MA, Rafiq MA, Noor A, Ali N, Ali G, Vincent JB, Ansar M. Khan MA, et al. BMC Med Genet. 2011 Apr 22;12:56. doi: 10.1186/1471-2350-12-56. BMC Med Genet. 2011. PMID: 21513506 Free PMC article. - Reduction of aberrant NF-κB signalling ameliorates Rett syndrome phenotypes in Mecp2-null mice.
Kishi N, MacDonald JL, Ye J, Molyneaux BJ, Azim E, Macklis JD. Kishi N, et al. Nat Commun. 2016 Jan 29;7:10520. doi: 10.1038/ncomms10520. Nat Commun. 2016. PMID: 26821816 Free PMC article. - COPI-TRAPPII activates Rab18 and regulates its lipid droplet association.
Li C, Luo X, Zhao S, Siu GK, Liang Y, Chan HC, Satoh A, Yu SS. Li C, et al. EMBO J. 2017 Feb 15;36(4):441-457. doi: 10.15252/embj.201694866. Epub 2016 Dec 21. EMBO J. 2017. PMID: 28003315 Free PMC article. - A trapper keeper for TRAPP, its structures and functions.
Yu S, Liang Y. Yu S, et al. Cell Mol Life Sci. 2012 Dec;69(23):3933-44. doi: 10.1007/s00018-012-1024-3. Epub 2012 Jun 6. Cell Mol Life Sci. 2012. PMID: 22669257 Free PMC article. Review.
References
- WHO . Mental health: new understanding, new hope. In: Murthy R.S., editor. World Health Report 2001. World Health Organization; Geneva: 2001. p. 35.
- Ropers H.H. Genetics of intellectual disability. Curr. Opin. Genet. Dev. 2008;18:241–250. - PubMed
- Ropers H.H. X-linked mental retardation: many genes for a complex disorder. Curr. Opin. Genet. Dev. 2006;16:260–269. - PubMed
- Najmabadi H., Motazacker M.M., Garshasbi M., Kahrizi K., Tzschach A., Chen W., Behjati F., Hadavi V., Nieh S.E., Abedini S.S. Homozygosity mapping in consanguineous families reveals extreme heterogeneity of non-syndromic autosomal recessive mental retardation and identifies 8 novel gene loci. Hum. Genet. 2007;121:43–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- G9900837/MRC_/Medical Research Council/United Kingdom
- G0700089/MRC_/Medical Research Council/United Kingdom
- HHMI/Howard Hughes Medical Institute/United States
- 2R01NS035129-12/NS/NINDS NIH HHS/United States
- R01 NS035129/NS/NINDS NIH HHS/United States
- HHSN268200782096C/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases