TOR-dependent control of autophagy: biting the hand that feeds - PubMed (original) (raw)
Review
TOR-dependent control of autophagy: biting the hand that feeds
Thomas P Neufeld. Curr Opin Cell Biol. 2010 Apr.
Abstract
Induction of autophagy in response to starvation is a highly conserved ability of eukaryotic cells, indicating a crucial and ancient role of this process in adapting to nutrient conditions. The target of rapamycin (TOR) pathway is major conduit for such signals, and in most cell types TOR activity is necessary and sufficient to suppress autophagy under favorable growth conditions. Recent studies have begun to reveal how TOR activity is regulated in response to nutritional cues, and are shedding new light on the mechanisms by which TOR controls the autophagic machinery. In addition, a variety of signals, stressors and pharmacological agents that induce autophagy independent of nutrient conditions have been identified. In some cases these signals appear to have been spliced into the core TOR pathway, whereas others are able to bypass the control mechanisms regulated by TOR. Increasing evidence is pointing to an important role for both positive and negative feedback loops in controlling this pathway, leading to an emerging view that TOR signaling not only regulates autophagy but is also highly sensitive to cellular rates of autophagy and other TOR-dependent processes.
Copyright 2009 Elsevier Ltd. All rights reserved.
Figures
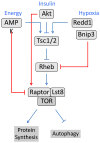
Figure 1. Mechanisms of signaling redundancy in the TOR pathway
Multiple inputs regulate activity of the TOR/Raptor/Lst8 complex (TORC1) through redundant upstream signaling pathways. Interactions leading to activation of a downstream component are indicated by arrows; those that inhibit are indicated by a bar. The canonical Tsc1/Tsc2-dependent TOR pathway is indicated by the blue lines. Recently described Tsc1/Tsc2-independent signals are shown in red. Decreases in cellular energy level (ATP/AMP ratio) cause phosphorylation of Raptor by the AMP-dependent protein kinase, leading to 14-3-3 mediated inhibition of TORC1 [20]. In response to insulin and other growth factors, Akt phosphorylates and inactivates PRAS40 (not shown), an inhibitor of TORC1 [–24]. Decreases in cellular oxygen levels lead to increased expression of hypoxia-induced genes, including Bnip3, which binds and inhibits the small GTPase Rheb [27].
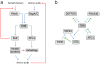
Figure 2. Mechanisms of feedback signaling in the TOR pathway
(a) Negative feedback mechanisms that limit activation of autophagy are shown in red. Protein degradation within the autolysosome raises the intracellular levels of amino acids, leading to activation of TOR through the RagA/RagC small GTPases and other nutrient signaling pathways. TOR activity is self-limited through an S6K-mediated inhibition of IRS1 (not shown), an essential component in the insulin/PI3K/Akt pathway upstream of Rheb. S6K is also involved in a self-limiting pathway in autophagy induction downstream of TOR, probably through its function in protein synthesis. Inhibition of TOR leads to both activation of Atg1 and inactivation of S6K; both of these kinases play positive roles in starvation-induced autophagy. (b) Feed-forward mechanisms in TOR signaling, indicated by green lines, may lead to amplification and stabilization of initially modest changes in TOR activity. DEPTOR, PRAS40 and Atg1 are each inhibited by TOR mediated phosphorylation and in turn inhibit TOR activity. In yeast, TOR mediates activation of Tap42, which leads to downregulation of the phosphatase Sit4 and decreased activity of the Tap42 inhibitor Tip41, resulting in further activation of Tap42.
Similar articles
- mTOR regulation of autophagy.
Jung CH, Ro SH, Cao J, Otto NM, Kim DH. Jung CH, et al. FEBS Lett. 2010 Apr 2;584(7):1287-95. doi: 10.1016/j.febslet.2010.01.017. Epub 2010 Jan 18. FEBS Lett. 2010. PMID: 20083114 Free PMC article. Review. - Direct induction of autophagy by Atg1 inhibits cell growth and induces apoptotic cell death.
Scott RC, Juhász G, Neufeld TP. Scott RC, et al. Curr Biol. 2007 Jan 9;17(1):1-11. doi: 10.1016/j.cub.2006.10.053. Curr Biol. 2007. PMID: 17208179 Free PMC article. - The Tor and PKA signaling pathways independently target the Atg1/Atg13 protein kinase complex to control autophagy.
Stephan JS, Yeh YY, Ramachandran V, Deminoff SJ, Herman PK. Stephan JS, et al. Proc Natl Acad Sci U S A. 2009 Oct 6;106(40):17049-54. doi: 10.1073/pnas.0903316106. Epub 2009 Sep 21. Proc Natl Acad Sci U S A. 2009. PMID: 19805182 Free PMC article. - Nutrient-dependent regulation of autophagy through the target of rapamycin pathway.
Chang YY, Juhász G, Goraksha-Hicks P, Arsham AM, Mallin DR, Muller LK, Neufeld TP. Chang YY, et al. Biochem Soc Trans. 2009 Feb;37(Pt 1):232-6. doi: 10.1042/BST0370232. Biochem Soc Trans. 2009. PMID: 19143638 Review. - TOR Signaling and Nutrient Sensing.
Dobrenel T, Caldana C, Hanson J, Robaglia C, Vincentz M, Veit B, Meyer C. Dobrenel T, et al. Annu Rev Plant Biol. 2016 Apr 29;67:261-85. doi: 10.1146/annurev-arplant-043014-114648. Epub 2016 Feb 22. Annu Rev Plant Biol. 2016. PMID: 26905651 Review.
Cited by
- Understanding the mechanisms of proteinuria: therapeutic implications.
Toblli JE, Bevione P, Di Gennaro F, Madalena L, Cao G, Angerosa M. Toblli JE, et al. Int J Nephrol. 2012;2012:546039. doi: 10.1155/2012/546039. Epub 2012 Jul 4. Int J Nephrol. 2012. PMID: 22844592 Free PMC article. - Programmed cell death in tumor immunity: mechanistic insights and clinical implications.
Wang M, Yu F, Zhang Y, Li P. Wang M, et al. Front Immunol. 2024 Jan 12;14:1309635. doi: 10.3389/fimmu.2023.1309635. eCollection 2023. Front Immunol. 2024. PMID: 38283351 Free PMC article. Review. - Target of rapamycin (TOR) regulates the response to low nitrogen stress via autophagy and hormone pathways in Malus hupehensis.
Li D, Ding Y, Cheng L, Zhang X, Cheng S, Ye Y, Gao Y, Qin Y, Liu Z, Li C, Ma F, Gong X. Li D, et al. Hortic Res. 2022 Jun 27;9:uhac143. doi: 10.1093/hr/uhac143. eCollection 2022. Hortic Res. 2022. PMID: 36072834 Free PMC article. - AATF: A new actor in the complex world of mTOR.
Fanciulli M. Fanciulli M. Mol Cell Oncol. 2015 Jul 15;3(1):e1040946. doi: 10.1080/23723556.2015.1040946. eCollection 2016 Jan. Mol Cell Oncol. 2015. PMID: 27308559 Free PMC article. - Autophagy and the Insulin-like Growth Factor (IGF) System in Colonic Cells: Implications for Colorectal Neoplasia.
Kasprzak A. Kasprzak A. Int J Mol Sci. 2023 Feb 11;24(4):3665. doi: 10.3390/ijms24043665. Int J Mol Sci. 2023. PMID: 36835075 Free PMC article. Review.
References
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. - PubMed
- Smith EM, Finn SG, Tee AR, Browne GJ, Proud CG. The tuberous sclerosis protein TSC2 is not required for the regulation of the mammalian target of rapamycin by amino acids and certain cellular stresses. J Biol Chem. 2005;280:18717–18727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources