De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease - PubMed (original) (raw)
De novo expression of podocyte proteins in parietal epithelial cells during experimental glomerular disease
Takamoto Ohse et al. Am J Physiol Renal Physiol. 2010 Mar.
Abstract
Studies have shown that certain cells of the glomerular tuft begin to express proteins considered unique to other cell types upon injury. Little is known about the response of parietal epithelial cells (PEC) to injury. To determine whether PECs change their phenotype upon injury to also express proteins traditionally considered podocyte specific, the following four models of glomerular disease were studied: the transforming growth factor (TGF)-beta1 transgenic mouse model of global glomerulosclerosis, the adriamycin model of focal segmental glomerulosclerosis (FSGS), the anti-glomerular basement membrane (GBM) model of crescentic glomerulonephritis, and the passive Heymann nephritis model of membranous nephropathy. Double immunostaining was performed with antibodies to podocyte-specific proteins (synaptopodin and Wilms' tumor 1) and antibodies to PEC specific proteins (paired box gene 8 and claudin-1). No double staining was detected in normal mice. In contrast, the results showed a statistical increase in the number of cells attached to Bowman basement membrane that were double-positive for both podocyte/PEC proteins in TGF-beta1 transgenic, anti-GBM, and membranous animals. Double-positive cells for both podocyte and PEC proteins were also statistically increased in the glomerular tuft in TGF-beta1 transgenic, anti-GBM, and FSGS mice. These results are consistent with glomerular cells coexpressing podocyte and PEC proteins in experimental glomerular disease, but not under normal circumstances.
Figures
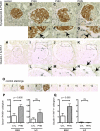
Fig. 1.
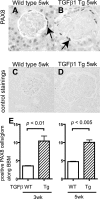
Paired box gene 8 (PAX8) staining in transforming growth factor (TGF)-β1 transgenic (Tg) mice. PAX8 staining was performed to evaluate the change in the number of parietal epithelial cells (PECs) in wild-type (WT; A and C) and TGF-β1 Tg mice (B and D). C and D: the primary antibody was omitted as a control. E: statistical analysis showed a significant increase in the number of PECs in TGF-β1 Tg mice at both 3 and 5 wk of age.
Fig. 2.
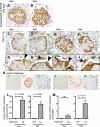
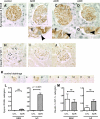
Double-positive cells for synaptopodin and PAX8 in TGF-β1 Tg mice. Double-positive cells for synaptopodin and PAX8 were evaluated in WT mice at 3 (A) and 5 wk (B) as well as in TGF-β1 Tg mice at 3 (C) and 5 wk (D_–_F). Higher magnification images are also shown (G_–_J). Arrows indicate double-positive cells along Bowman's basement membrane (BBM; G_–_J). Black arrowheads show double-positive cells in tuft, while white arrowheads show single-positive (synaptopodin-positive, PAX8-negative) cells (G_–_J). K: images of controls in which primary antibodies were omitted. Images from WT mice with no primary antibody (K1), with synaptopodin only (K2), and PAX8 only (K3) and from TGF-β1 Tg mice with no primary antibody (K4), with synaptopodin only (K5), and PAX8 only (K6) are shown. Double-positive cells were quantified, and results are shown in L and M. Double-positive cells for synaptopodin and PAX8 in the glomerular tuft significantly increased (L, 3 and 5 wk) as well as along BBM (M, 5 wk). Synpo, synaptopodin.
Fig. 3.
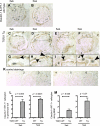
Double-positive cells for claudin-1 and Wilms' tumor 1 (WT-1) in TGF-β1 Tg mice. Double-positive cells for claudin-1 and WT-1 were evaluated in WT mice at 3 (A) and 5 wk (B) as well as in TGF-β1 Tg mice at 3 (C) and 5 wk (D_–_F). Higher magnification images are also shown (G_–_J). Arrows indicate the double-positive cells along BBM (G_–_J), and arrowheads show the double-positive cells in tuft (G_–_J). K: images of controls in which primary antibodies were omitted. Images from WT mice with no primary antibody (K1), with claudin-1 only (K2), and WT-1 only (K3) and from TGF-β1 Tg mice with no primary antibody (K4), with claudin-1 only (K5), and WT-1 only (K6) are shown. Double-positive cells were quantified, and results are shown in L and M. Double-positive cells for claudin-1 and WT-1 in tuft significantly increased (L, 3 and 5 wk) as well as along BBM (M, 3 and 5 wk).
Fig. 4.
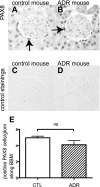
PAX8 staining in adriamycin (ADR) nephropathy mice. PAX8 was stained to evaluate the change in the number of PECs in control (A and C) and ADR mice (B and D). C and D: primary antibody-omitted staining control. E: no statistical difference was observed between ADR nephropathy mice and control mice.
Fig. 5.
Double-positive cells in ADR nephropathy mice. Double-positive cells for synaptopodin and PAX8 as well as claudin-1 and WT-1 were evaluated in ADR mice 8 wk after disease induction. Double-staining images of control (A) and ADR mice (B_–_D: full images of glomeruli; E_–_G: high magnification) for synaptopodin and PAX8 are shown. Arrowheads indicate the double-positive cells in tuft (E_–_G). Double-staining images are shown for control (H) and ADR mice (I and J) for claudin-1 and WT-1. Most of cells did not show double-positive signals. K: images of controls in which primary antibodies were omitted for synaptopodin and PAX8 (K1–6) and for claudin-1 and WT-1 (K7–12). Images from control mice with no primary antibody (K1), with synaptopodin only (K2), and PAX8 only (K3); from ADR mice with no primary antibody (K4), with synaptopodin only (K5), and PAX8 only (K6); from control mice with no primary antibody (K7), with claudin-1 only (K8), and WT-1 only (K9); and from ADR mice with no primary antibody (K10), with claudin-1 only (K11), and WT-1 only (K12) are shown. Double-positive cells were quantified, and results are shown in L and M. Double-positive cells for synaptopodin and PAX8 in the tuft significantly increased (L), but no statistical difference was observed in cells along BBM (L). No difference was observed in double staining of claudin-1 and WT-1 in either cells along BBM or in tuft.
Fig. 6.
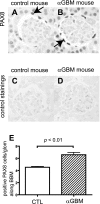
PAX8 staining in anti-glomerular basement membrane (GBM) nephritis mice. PAX8 was stained to evaluate the change in the number of PECs in control mice (A and C) and anti-GBM mice (B and D) 9 days after disease induction. C and D: primary antibody-omitted staining control. E: statistical analysis showed a significant increase in the number of PECs in anti-GBM mice.
Fig. 7.
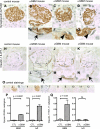
Double positive cells in anti-GBM nephritis mice. Double-positive cells for synaptopodin and PAX8 as well as claudin-1 and WT-1 were evaluated in anti-GBM nephritis mice 9 days after disease induction. Double-staining images of control (A) and anti-GBM mice (B_–_D: full images of glomeruli, E_–_G: high magnification) for synaptopodin and PAX8 are shown. Arrow indicates the double-positive cells along BBM (E), and arrowheads indicate the double-positive cells in tuft (F and G). Double-staining images of control (H) and anti-GBM mice (I_–_K: full images of glomeruli, L_–_N: high magnification) for claudin-1 and WT-1 are shown. Arrows indicate the double-positive cells along BBM (L_–_N). O: images of controls in which primary antibodies were omitted for synaptopodin and PAX8 (O1–6), and for claudin-1 and WT-1 (O7–12). Images from control mice with no primary antibody (O1), with synaptopodin only (O2), and PAX8 only (O3); from anti-GBM mice with no primary antibody (O4), with synaptopodin only (O5), PAX8 only (O6); from control mice with no primary antibody (O7), with claudin-1 only (O8), and WT-1 only (O9); and from anti-GBM mice with no primary antibody (O10), with claudin-1 only (O11), and WT-1 only (O12) are shown. Double-positive cells were quantified, and results are shown in P and Q. Double-positive cells for synaptopodin and PAX8 significantly increased in tuft and along BBM (P). Double-positive cells for claudin-1 and WT-1 also increased significantly along BBM, but an increase was not observed in tuft (Q).
Fig. 8.
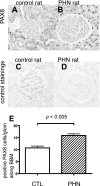
PAX8 staining in passive Heymann nephritis (PHN) rats. PAX8 was stained to evaluate the change in the number of PECs in control rats (A and C) and PHN rats (B and D) at day 110. C and D: primary antibody-omitted staining control. E: statistical analysis showed a significant increase in the number of PECs in PHN rats.
Fig. 9.
Double-positive cells in PHN rats. Double-positive cells for synaptopodin and PAX8 as well as claudin-1 and WT-1 were evaluated in PHN rats at day 110. Double-staining images of control (A) and PHN rats (B_–_D: full images of glomeruli, E_–_G: high magnification) for synaptopodin and PAX8 are shown. Arrows indicate the double-positive cells along BBM (E_–_G). Double-staining images of control (H) and PHN rats (I_–_K: full images of glomeruli, L_–_N: high magnification) for claudin-1 and WT-1 are shown. Arrows indicate the double-positive cells along BBM (L_–_N). O: images of controls in which primary antibodies were omitted for synaptopodin and PAX8 (O1–6), and for claudin-1 and WT-1 (O7–12). Images from control rats with no primary antibody (O1), with synaptopodin only (O2), and PAX8 only (O3); from PHN rats with no primary antibody (O4), with synaptopodin only (O5), and PAX8 only (O6); from control mice with no primary antibody (O7), with claudin-1 only (O8), and WT-1 only (O9); and from PHN rats with no primary antibody (O10), with claudin-1 only (O11), and WT-1 only (O12) are shown. Double-positive cells were quantified, and results are shown in P and Q. Double-positive cells for synaptopodin and PAX8 significantly increased in cells along BBM (P). Also, double-positive cells for claudin-1 and WT-1 increased significantly along BBM (Q). However, an increase was not observed in cells in tuft for either synaptopodin and PAX8 (P) or claudin-1 and WT-1 (Q).
Similar articles
- New insights into glomerular parietal epithelial cell activation and its signaling pathways in glomerular diseases.
Su H, Chen S, He FF, Wang YM, Bondzie P, Zhang C. Su H, et al. Biomed Res Int. 2015;2015:318935. doi: 10.1155/2015/318935. Epub 2015 Mar 19. Biomed Res Int. 2015. PMID: 25866774 Free PMC article. Review. - Retinoids augment the expression of podocyte proteins by glomerular parietal epithelial cells in experimental glomerular disease.
Zhang J, Pippin JW, Vaughan MR, Krofft RD, Taniguchi Y, Romagnani P, Nelson PJ, Liu ZH, Shankland SJ. Zhang J, et al. Nephron Exp Nephrol. 2012;121(1-2):e23-37. doi: 10.1159/000342808. Epub 2012 Oct 25. Nephron Exp Nephrol. 2012. PMID: 23107969 Free PMC article. - CD44 is required for the pathogenesis of experimental crescentic glomerulonephritis and collapsing focal segmental glomerulosclerosis.
Eymael J, Sharma S, Loeven MA, Wetzels JF, Mooren F, Florquin S, Deegens JK, Willemsen BK, Sharma V, van Kuppevelt TH, Bakker MA, Ostendorf T, Moeller MJ, Dijkman HB, Smeets B, van der Vlag J. Eymael J, et al. Kidney Int. 2018 Mar;93(3):626-642. doi: 10.1016/j.kint.2017.09.020. Epub 2017 Dec 21. Kidney Int. 2018. PMID: 29276101 - Urinary podocyte and TGF-β1 mRNA as markers for disease activity and progression in anti-glomerular basement membrane nephritis.
Fukuda A, Minakawa A, Sato Y, Iwakiri T, Iwatsubo S, Komatsu H, Kikuchi M, Kitamura K, Wiggins RC, Fujimoto S. Fukuda A, et al. Nephrol Dial Transplant. 2017 Nov 1;32(11):1818-1830. doi: 10.1093/ndt/gfx047. Nephrol Dial Transplant. 2017. PMID: 28419296 Free PMC article. - Mechanisms and consequences of TGF-ß overexpression by podocytes in progressive podocyte disease.
Lee HS. Lee HS. Cell Tissue Res. 2012 Jan;347(1):129-40. doi: 10.1007/s00441-011-1169-7. Epub 2011 May 4. Cell Tissue Res. 2012. PMID: 21541658 Free PMC article. Review.
Cited by
- New insights into glomerular parietal epithelial cell activation and its signaling pathways in glomerular diseases.
Su H, Chen S, He FF, Wang YM, Bondzie P, Zhang C. Su H, et al. Biomed Res Int. 2015;2015:318935. doi: 10.1155/2015/318935. Epub 2015 Mar 19. Biomed Res Int. 2015. PMID: 25866774 Free PMC article. Review. - Claudin 1 and nephrin label cellular crescents in diabetic glomerulosclerosis.
Gaut JP, Hoshi M, Jain S, Liapis H. Gaut JP, et al. Hum Pathol. 2014 Mar;45(3):628-35. doi: 10.1016/j.humpath.2013.10.030. Epub 2013 Nov 12. Hum Pathol. 2014. PMID: 24529330 Free PMC article. - Compound effects of aging and experimental FSGS on glomerular epithelial cells.
Schneider RR, Eng DG, Kutz JN, Sweetwyne MT, Pippin JW, Shankland SJ. Schneider RR, et al. Aging (Albany NY). 2017 Feb 17;9(2):524-546. doi: 10.18632/aging.101176. Aging (Albany NY). 2017. PMID: 28222042 Free PMC article. - Krüppel-Like Factor 15 Mediates Glucocorticoid-Induced Restoration of Podocyte Differentiation Markers.
Mallipattu SK, Guo Y, Revelo MP, Roa-Peña L, Miller T, Ling J, Shankland SJ, Bialkowska AB, Ly V, Estrada C, Jain MK, Lu Y, Ma'ayan A, Mehrotra A, Yacoub R, Nord EP, Woroniecki RP, Yang VW, He JC. Mallipattu SK, et al. J Am Soc Nephrol. 2017 Jan;28(1):166-184. doi: 10.1681/ASN.2015060672. Epub 2016 Jun 10. J Am Soc Nephrol. 2017. PMID: 27288011 Free PMC article. - Glomerular parietal epithelial cells contribute to adult podocyte regeneration in experimental focal segmental glomerulosclerosis.
Eng DG, Sunseri MW, Kaverina NV, Roeder SS, Pippin JW, Shankland SJ. Eng DG, et al. Kidney Int. 2015 Nov;88(5):999-1012. doi: 10.1038/ki.2015.152. Epub 2015 May 20. Kidney Int. 2015. PMID: 25993321 Free PMC article.
References
- Alpers CE, Hudkins KL, Gown AM, Johnson RJ. Enhanced expression of “muscle-specific” actin in glomerulonephritis. Kidney Int 41: 1134–1142, 1992 - PubMed
- Bariety J, Hill GS, Mandet C, Irinopoulou T, Jacquot C, Meyrier A, Bruneval P. Glomerular epithelial-mesenchymal transdifferentiation in pauci-immune crescentic glomerulonephritis. Nephrol Dial Transplant 18: 1777–1784, 2003 - PubMed
- Bariety J, Mandet C, Hill GS, Bruneval P. Parietal podocytes in normal human glomeruli. J Am Soc Nephrol 17: 2770–2780, 2006 - PubMed
- Barisoni L, Kriz W, Mundel P, D'Agati V. The dysregulated podocyte phenotype: a novel concept in the pathogenesis of collapsing idiopathic focal segmental glomerulosclerosis, and HIV-associated nephropathy. J Am Soc Nephrol 10: 51–61, 1999 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources